Abstract
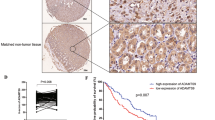
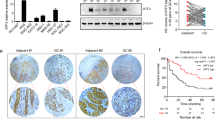
Using genome-wide promoter methylation analysis, we identified a disintegrin-like and metalloprotease with thrombospondin type 1 motif 9 (ADAMTS9) is methylated in cancer. We aim to clarify its epigenetic inactivation, biological function and clinical implication in gastric cancer. ADAMTS9 was silenced in 6 out of 8 gastric cancer cell lines. The loss of ADAMTS9 expression was regulated by promoter hypermethylation and could be restored by demethylation agent. Ectopic expression of ADAMTS9 in gastric cancer cell lines (AGS, BGC823) inhibited cell growth curve in both the cell lines (P<0.0001), suppressed colony formation (P<0.01) and induced apoptosis (P<0.001 in AGS, P<0.01 in BGC823). Moreover, conditioned culture medium from ADAMTS9-transfected cell lines significantly disrupted the human umbilical vein endothelial cell tube formation capacity on Matrigel (P<0.01 in AGS, P<0.001 in BGC823). The in vivo growth of ADAMTS9 cells in nude mice was also markedly diminished after stable expression of ADAMTS9 (P<0.001). On the other hand, ADAMTS9 knockdown promoted cell proliferation (P<0.001). We further revealed that ADAMTS9 inhibited tumor growth by blocking activation of Akt and its downstream target the mammalian target of rapamycin (mTOR). ADAMTS9 also reduced phosphorylation of mTOR downstream targets p70 ribosomal S6 kinase, eIF4E-binding protein and downregulated hypoxia-inducible factor-1α. Therefore, this is the first demonstration that ADAMTS9 is a critical tumor suppressor of gastric cancer progression at least in part through suppression of oncogenic AKT/mTOR signaling. Moreover, promoter methylation of ADAMTS9 was detected in 29.2% (21/72) of primary gastric tumors. Multivariate analysis showed that patients with ADAMTS9 methylation had a poorer overall survival (relative risk (RR)=2.788; 95% confidence interval, 1.474–5.274; P=0.002). Kaplan–Meier survival curves showed that ADAMTS9 methylation was significantly associated with shortened survival in gastric cancer patients (P=0.001, log-rank test). In conclusion, ADAMTS9 acts as a functional tumor suppressor in gastric cancer through inhibiting oncogenic AKT/mTOR signaling pathway. Methylation of ADAMTS9 is an independent prognostic factor of gastric cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- 7-AAD:
-
7-amino-actinomycin
- 5-Aza:
-
5-Aza-2′-deoxycytidine
- ADAMTS9:
-
a disintegrin-like and metalloprotease with thrombospondin type 1 motif 9
- BCL-2:
-
B-cell CLL/lymphoma 2
- BGS:
-
bisulfite genomic sequencing
- CDK4:
-
cyclin-dependent kinase 4
- eIF4E:
-
eukaryotic translation initiation factor 4E
- 4E-BP1:
-
eIF4E-binding protein
- EGF:
-
epidermal growth factor
- GLUT-1:
-
glucose transporter 1
- HIF1α:
-
hypoxia-inducible factor 1α
- HK2:
-
hexokinase 2
- HUVEC:
-
human umbilical vein endothelial cell
- MSP:
-
methylation specific PCR
- mTOR:
-
the mammalian target of rapamycin
- PARP:
-
nuclear enzyme poly (ADP-ribose) polymerase
- PGK1:
-
phospho-glycerate kinase 1
- PI3K:
-
phosphoinositide 3-kinase
- PCNA:
-
proliferating cell nuclear antigen
- VEGFA:
-
vascular endothelial growth factor A.
References
Yu J, Cheng YY, Tao Q, Cheung KF, Lam CN, Geng H et al. Methylation of protocadherin 10, a novel tumor suppressor, is associated with poor prognosis in patients with gastric cancer. Gastroenterology 2009; 136: 640–651.
Yu J, Tao Q, Cheng YY, Lee KY, Ng SS, Cheung KF et al. Promoter methylation of the Wnt/beta-catenin signaling antagonist Dkk-3 is associated with poor survival in gastric cancer. Cancer 2009; 115: 49–60.
Cheung KF, Lam CN, Wu K, Ng EK, Chong WW, Cheng AS et al. Characterization of the gene structure, functional significance, and clinical application of RNF180, a novel gene in gastric cancer. Cancer 2012; 118: 947–959.
Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK et al. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res 2005; 11: 6442–6449.
Liu W, Li X, Chu ES, Go MY, Xu L, Zhao G et al. Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology 2011; 53: 843–853.
Lo PH, Lung HL, Cheung AK, Apte SS, Chan KW, Kwong FM et al. Extracellular protease ADAMTS9 suppresses esophageal and nasopharyngeal carcinoma tumor formation by inhibiting angiogenesis. Cancer Res 2010; 70: 5567–5576.
Lo PH, Leung AC, Kwok CY, Cheung WS, Ko JM, Yang LC et al. Identification of a tumor suppressive critical region mapping to 3p14.2 in esophageal squamous cell carcinoma and studies of a candidate tumor suppressor gene, ADAMTS9. Oncogene 2007; 26: 148–157.
Lung HL, Lo PH, Xie D, Apte SS, Cheung AK, Cheng Y et al. Characterization of a novel epigenetically-silenced, growth-suppressive gene, ADAMTS9, and its association with lymph node metastases in nasopharyngeal carcinoma. Int J Cancer 2008; 123: 401–408.
Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S et al. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem 2003; 278: 9503–9513.
Clark ME, Kelner GS, Turbeville LA, Boyer A, Arden KC, Maki RA . ADAMTS9 a novel member of the ADAM-TS/ metallospondin gene family. Genomics 2000; 67: 343–350.
Jungers KA, Le Goff C, Somerville RP, Apte SS . Adamts9 is widely expressed during mouse embryo development. Gene Expr Patterns 2005; 5: 609–617.
Koo BH, Coe DM, Dixon LJ, Somerville RP, Nelson CM, Wang LW et al. ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. Am J Pathol 2010; 176: 1494–1504.
Zoncu R, Efeyan A, Sabatini DM . mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011; 12: 21–35.
Yecies JL, Manning BD . mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med 2011; 89: 221–228.
Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL . HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 2001; 21: 3995–4004.
Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 2004; 10: 594–601.
Jin Z, El-Deiry WS . Overview of cell death signaling pathways. Cancer Biol Ther 2005; 4: 139–163.
Luo J, Manning BD, Cantley LC . Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 2003; 4: 257–262.
Vivanco I, Sawyers CL . The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501.
Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J . mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 2004; 24: 200–216.
Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J . Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 2003; 13: 1259–1268.
Ma XM, Blenis J . Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2009; 10: 307–318.
Averous J, Fonseca BD, Proud CG . Regulation of cyclin D1 expression bymTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene 2008; 27: 1106–1113.
Asnaghi L, Calastretti A, Bevilacqua A, D’Agnano I, Gatti G, Canti G et al. Bcl-2 phosphorylation and apoptosis activated by damaged microtubules require mTOR and are regulated by Akt. Oncogene 2004; 23: 5781–5791.
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL . Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 2011; 11: 558–572.
Cicenas J, Valius M . The CDK inhibitors in cancer research and therapy. J Cancer Res Clin Oncol 2011; 137: 1409–1418.
Troiano L, Ferraresi R, Lugli E, Nemes E, Roat E, Nasi M et al. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nat Protoc 2007; 2: 2719–2727.
Adams JM, Cory S . The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007; 26: 1324–1337.
Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S et al. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem 2003; 278: 9503–9513.
Liu YJ, Xu Y, Yu Q . Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene 2006; 25: 2452–2467.
Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS et al. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol 2010; 29: 304–316.
Leung WK, Man EP, Yu J, Go MY, To KF, Yamaoka Y et al. Effects of helicobacter pylori eradication on methylation status of e-cadherin gene in noncancerous stomach. Clin Cancer Res 2006; 12: 3216–3221.
Sepulveda AR, Yao Y, Yan W, Park DI, Kim JJ, Gooding W et al. CpG methylation and reduced expression of O6-methylguanine DNA methyltransferase is associated with Helicobacter pylori infection. Gastroenterology 2010; 138: 1836–1844.
Koo BH, Longpré JM, Somerville RP, Alexander JP, Leduc R, Apte SS . Cell-surface processing of pro-ADAMTS9 by furin. J Biol Chem 2006; 281: 12485–12494.
Acknowledgements
This project was supported by National Basic Research Program of China (973 Program, 2010CB529305); Shenzhen Basic Research Program (JC20110520111A); Research Grants Council RGC CERG CUHK (473008); Group Research Scheme CUHK (3110043); CUHK Focused Investment Gant (1903026), and RFCID (10090942, 11100022).
Author contributions: WD, SW, QZ, XL, KFC and JC performed the experiments; QT, participated in initial conception, technical and material support; ZC provided technical and material support; WD and SW analyzed the data and drafted the paper; JF and EKON provided material support; JJYS supervised and commented on the study; JY designed, supervised study and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Du, W., Wang, S., Zhou, Q. et al. ADAMTS9 is a functional tumor suppressor through inhibiting AKT/mTOR pathway and associated with poor survival in gastric cancer. Oncogene 32, 3319–3328 (2013). https://doi.org/10.1038/onc.2012.359
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.359
Keywords
This article is cited by
-
ADAMTS9-AS1 inhibits tumor growth and drug resistance in clear cell renal cell carcinoma via recruiting HuR to enhance ADAMTS9 mRNA stability
Cancer Nanotechnology (2023)
-
MicroRNA profiling of royal jelly extracellular vesicles and their potential role in cell viability and reversing cell apoptosis
Functional & Integrative Genomics (2023)
-
ADAMTS9-AS1 Long Non‑coding RNA Sponges miR‑128 and miR-150 to Regulate Ras/MAPK Signaling Pathway in Glioma
Cellular and Molecular Neurobiology (2023)
-
Signaling pathways and therapeutic interventions in gastric cancer
Signal Transduction and Targeted Therapy (2022)
-
DNMT3A-mediated silence in ADAMTS9 expression is restored by RNF180 to inhibit viability and motility in gastric cancer cells
Cell Death & Disease (2021)