Abstract
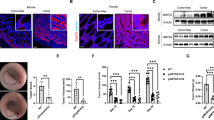
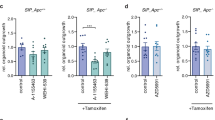
Reduced expression of the pro-apoptotic protein SMAC (second mitochondria-derived activator of caspase) has been reported to correlate with cancer progression, while its significance and underlying mechanisms are poorly understood. In this study, we investigated the role of SMAC in intestinal tumorigenesis using both human samples and animal models. Decreased SMAC expression was found to correlate with increased cIAP2 expression and higher grades of human colon cancer. In mice, SMAC deficiency significantly increased the incidence and size of colon tumors induced by azoxymethane (AOM)/dextran sulfate sodium salt (DSS), and highly enriched β-catenin hot spot mutations. SMAC deficiency also significantly increased the incidence of spontaneous intestinal polyps in APCMin/+ mice. Loss of SMAC in mice led to elevated levels of cIAP1 and cIAP2, increased proliferation and activation of the NF-κB p65 subunit in normal and tumor tissues. Unexpectedly, SMAC deficiency had little effect on the incidence of precursor lesions, or apoptosis induced by AOM or DSS, or in established tumors in mice. Furthermore, SMAC knockout enhanced TNFα-mediated NF-κB activation via cIAP2 in HCT 116 colon cancer cells. These results demonstrate an essential and apoptosis-independent function of SMAC in tumor suppression and provide new insights into the biology and targeting of colon cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- SMAC:
-
second mitochondria-derived activator of caspases
- IAPs:
-
inhibitors of apoptosis proteins
- BrdU:
-
5-bromodeoxyuridine
- TUNEL:
-
terminal deoxynucleotidyl transferase–mediated deoxyuridinetriphosphate nick end labeling
- WT:
-
wild type
- KO:
-
knockout
- AOM:
-
azoxymethane
- DSS:
-
dextran sulfate sodium salt
- ACF:
-
aberrant crypt foci
- NSAID:
-
nonsteroidal anti-inflammatory drugs
- NF-κB:
-
nuclear factor kappa-light-chain-enhancer of activated B cells
- GSK-3β:
-
glycogen synthase kinase 3 beta
- TRAIL:
-
tumor necrosis factor-related apoptosis inducing ligand
- TNFα:
-
tumor necrosis factor alpha
References
Du C, Fang M, Li Y, Li L, Wang X . Smac a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102: 33–42.
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000; 102: 43–53.
Gyrd-Hansen M, Meier P . IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer 2010; 10: 561–574.
Yu J, Wang P, Ming L, Wood MA, Zhang L . SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene 2007; 26: 4189–4198.
Sun Q, Zheng X, Zhang L, Yu J . Smac modulates chemosensitivity in head and neck cancer cells through the mitochondrial apoptotic pathway. Clin Cancer Res 2011; 17: 2361–2372.
Kohli M, Yu J, Seaman C, Bardelli A, Kinzler KW, Vogelstein B et al. SMAC/Diablo-dependent apoptosis induced by nonsteroidal antiinflammatory drugs (NSAIDs) in colon cancer cells. Proc Natl Acad Sci USA 2004; 101: 16897–16902.
Bank A, Wang P, Du C, Yu J, Zhang L . SMAC mimetics sensitize nonsteroidal anti-inflammatory drug-induced apoptosis by promoting caspase-3-mediated cytochrome c release. Cancer Res 2008; 68: 276–284.
Fulda S, Wick W, Weller M, Debatin KM . Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med 2002; 8: 808–815.
Okada H, Suh WK, Jin J, Woo M, Du C, Elia A et al. Generation and characterization of Smac/DIABLO-deficient mice. Mol Cell Biol 2002; 22: 3509–3517.
Martinez-Ruiz G, Maldonado V, Ceballos-Cancino G, Grajeda JP, Melendez-Zajgla J . Role of Smac/DIABLO in cancer progression. J Exp Clin Cancer Res 2008; 27: 48.
Xu Y, Zhou L, Huang J, Liu F, Yu J, Zhan Q et al. Role of smac in determining the chemotherapeutic response of esophageal squamous cell carcinoma. Clin Cancer Res 2011; 17: 5412–5422.
Endo K, Kohnoe S, Watanabe A, Tashiro H, Sakata H, Morita M et al. Clinical significance of Smac/DIABLO expression in colorectal cancer. Oncol Rep 2009; 21: 351–355.
LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG . IAP-targeted therapies for cancer. Oncogene 2008; 27: 6252–6275.
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007; 131: 669–681.
Gaither A, Porter D, Yao Y, Borawski J, Yang G, Donovan J et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res 2007; 67: 11493–11498.
Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 2007; 131: 682–693.
Wang L, Du F, Wang X . TNF alpha induces two distinct caspase-8 activation pathways. Cell 2008; 133: 693–703.
Krajewska M, Krajewski S, Banares S, Huang X, Turner B, Bubendorf L et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res 2003; 9: 4914–4925.
Qiu W, Carson-Walter EB, Kuan SF, Zhang L, Yu J . PUMA suppresses intestinal tumorigenesis in mice. Cancer Res. 2009; 69: 4999–5006.
Stevens RG, Swede H, Rosenberg DW . Epidemiology of colonic aberrant crypt foci: review and analysis of existing studies. Cancer Lett 2007; 252: 171–183.
Clevers H . Wnt/beta-catenin signaling in development and disease. Cell 2006; 127: 469–480.
Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K . Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis 2000; 21: 1117–1120.
Hirose Y, Yoshimi N, Makita H, Hara A, Tanaka T, Mori H . Early alterations of apoptosis and cell proliferation in azoxymethane-initiated rat colonic epithelium. Jpn J Cancer Res 1996; 87: 575–582.
Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ et al. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest 2011; 121: 1722–1732.
Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 1992; 256: 668–670.
Qiu W, Wang X, Leibowitz B, Liu H, Barker N, Okada H et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci USA 2010; 107: 20027–20032.
Petersen SL, Peyton M, Minna JD, Wang X . Overcoming cancer cell resistance to Smac mimetic induced apoptosis by modulating cIAP-2 expression. Proc Natl Acad Sci USA 2010; 107: 11936–11941.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Mace PD, Smits C, Vaux DL, Silke J, Day CL . Asymmetric recruitment of cIAPs by TRAF2. J Mol Biol 2010; 400: 8–15.
Conze DB, Albert L, Ferrick DA, Goeddel DV, Yeh WC, Mak T et al. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol 2005; 25: 3348–3356.
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004; 118: 285–296.
Quante M, Wang TC . Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology 2008; 23: 350–359.
Grivennikov SI, Greten FR, Karin M . Immunity, inflammation, and cancer. Cell 2010; 140: 883–899.
Vlantis K, Wullaert A, Sasaki Y, Schmidt-Supprian M, Rajewsky K, Roskams T et al. Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J Clin Invest 2011; 121: 2781–2793.
Rakoff-Nahoum S, Medzhitov R . Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 2007; 317: 124–127.
Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 2008; 118: 560–570.
Guillen-Ahlers H, Suckow MA, Castellino FJ, Ploplis VA . Fas/CD95 deficiency in ApcMin/+ mice increases intestinal tumor burden. PLoS One 2010; 5: e9070.
Fingleton B, Carter KJ, Matrisian LM . Loss of functional Fas ligand enhances intestinal tumorigenesis in the Min mouse model. Cancer Res 2007; 67: 4800–4806.
Geserick P, Hupe M, Moulin M, Wong WW, Feoktistova M, Kellert B et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol 2009; 187: 1037–1054.
Yu J, Zhang L . Apoptosis in human cancer cells. Curr Opin Oncol 2004; 16: 19–24.
Leibowitz B, Yu J . Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol Ther 2010; 9: 417–422.
Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 2008; 30: 689–700.
Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA 2008; 105: 11778–11783.
Wu B, Qiu W, Wang P, Yu H, Cheng T, Zambetti GP et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut 2007; 56: 645–654.
Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2008; 2: 576–583.
Leibowitz BJ, Qiu W, Liu H, Cheng T, Zhang L, Yu J . Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol Cancer Res 2011; 9: 616–625.
Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 2003; 124: 762–777.
Yue W, Dacic S, Sun Q, Landreneau R, Guo M, Zhou W et al. Frequent inactivation of RAMP2, EFEMP1 and Dutt1 in lung cancer by promoter hypermethylation. Clin Cancer Res 2007; 13: 4336–4344.
Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B . PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 2001; 7: 673–682.
Wang P, Qiu W, Dudgeon C, Liu H, Huang C, Zambetti GP et al. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ 2009; 16: 1192–1202.
Acknowledgements
We thank Monica E Buchanan, Matthew F Brown and other members of Zhang and Yu labs for helpful discussion and critical reading, and Laurice A Vance-Carr for editorial assistance. This work is supported in part by NIH grants CA129829, UO1-DK085570, American Cancer Society grant RGS-10-124-01-CCE and FAMRI (JY), NIH grants CA106348, CA121105, and American Cancer Society grant RSG-07-156-01-CNE (LZ). This project used the UPCI shared glassware, animal, and cell and tissue imaging facilities that were supported in part by award P30CA047904.
Author contributions: WQ, HL, AS and QS performed the experiments and analyzed the data. HW analyzed the data. WQ, LZ and JY designed the experiments, analyzed the data and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Qiu, W., Liu, H., Sebastini, A. et al. An apoptosis-independent role of SMAC in tumor suppression. Oncogene 32, 2380–2389 (2013). https://doi.org/10.1038/onc.2012.265
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.265
Keywords
This article is cited by
-
A critical role for cellular inhibitor of protein 2 (cIAP2) in colitis-associated colorectal cancer and intestinal homeostasis mediated by the inflammasome and survival pathways
Mucosal Immunology (2016)
-
Role of Apoptosis in Colon Cancer Biology, Therapy, and Prevention
Current Colorectal Cancer Reports (2013)