Abstract
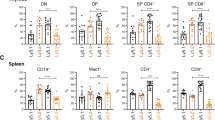
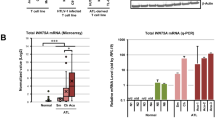
AVEN has been identified as an inhibitor of apoptosis, which binds to the adaptor protein, APAF-1, and thereby prevents apoptosome formation and mitochondrial apoptosis. Recent data have demonstrated high expression levels of AVEN messenger RNA in acute leukemias as well as a positive correlation between AVEN mRNA overexpression and poor prognosis in childhood acute lymphoblastic leukemia. On the basis of these data, we investigated the potential involvement of AVEN in tumorigenesis. First, we confirmed the overexpression of AVEN in T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) patient samples. We then established a transgenic mouse model with T-cell-specific overexpression of AVEN, with which we demonstrated the oncogenic cooperation of AVEN with heterozygous loss of p53. Finally, we used a subcutaneous xenograft mouse model to show that AVEN knockdown in the T-ALL cell lines, MOLT-4 and CCRF-CEM, and in the acute myeloblastic leukemia cell line, Kasumi-1, leads to a halt in tumor growth owing to the increased apoptosis and decreased proliferation of tumor cells. Collectively, our data demonstrate that the anti-apoptotic molecule, AVEN, functions as an oncoprotein in hematopoietic neoplasms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000; 100: 57–70.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Xu G, Shi Y . Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res 2007; 17: 759–771.
Ozoren N, El-Deiry WS . Cell surface Death Receptor signaling in normal and cancer cells. Semin Cancer Biol 2003; 13: 135–147.
Vangestel C, Van de Wiele C, Mees G, Peeters M . Forcing cancer cells to commit suicide. Cancer Biother Radiopharm 2009; 24: 395–407.
Brenner D, Mak TW . Mitochondrial cell death effectors. Curr Opin Cell Biol 2009; 21: 871–877.
Hajra KM, Liu JR . Apoptosome dysfunction in human cancer. Apoptosis 2004; 9: 691–704.
Ledgerwood EC, Morison IM . Targeting the apoptosome for cancer therapy. Clin Cancer Res 2009; 15: 420–424.
Bao Q, Shi Y . Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ 2007; 14: 56–65.
Bratton SB, Salvesen GS . Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci 2010; 123 (Part 19): 3209–3214.
Chau BN, Cheng EH, Kerr DA, Hardwick JM . Aven, a novel inhibitor of caspase activation, binds Bcl-xL and Apaf-1. Mol Cell 2000; 6: 31–40.
Esmaili AM, Johnson EL, Thaivalappil SS, Kuhn HM, Kornbluth S, Irusta PM . Regulation of the ATM-activator protein Aven by CRM1-dependent nuclear export. Cell Cycle 2010; 9: 3913–3920.
Guo JY, Yamada A, Kajino T, Wu JQ, Tang W, Freel CD et al. Aven-dependent activation of ATM following DNA damage. Curr Biol 2008; 18: 933–942.
Paydas S, Tanriverdi K, Yavuz S, Disel U, Sahin B, Burgut R . Survivin and aven: two distinct antiapoptotic signals in acute leukemias. Ann Oncol 2003; 14: 1045–1050.
Choi J, Hwang YK, Sung KW, Kim DH, Yoo KH, Jung HL et al. Aven overexpression: association with poor prognosis in childhood acute lymphoblastic leukemia. Leuk Res 2006; 30: 1019–1025.
Burkhardt B . Paediatric lymphoblastic T-cell leukaemia and lymphoma: one or two diseases? Br J Haematol 2010; 149: 653–668.
Cleaver AL, Beesley AH, Firth MJ, Sturges NC, O’Leary RA, Hunger SP et al. Gene-based outcome prediction in multiple cohorts of pediatric T-cell acute lymphoblastic leukemia: a Children's Oncology Group study. Mol Cancer 2010; 9: 105.
de Leval L, Bisig B, Thielen C, Boniver J, Gaulard P . Molecular classification of T-cell lymphomas. Crit Rev Oncol Hematol 2009; 72: 125–143.
Paolini S, Gazzola A, Sabattini E, Bacci F, Pileri S, Piccaluga PP . Pathobiology of acute lymphoblastic leukemia. Semin Diagn Pathol 2011; 28: 124–134.
Riz I, Hawley TS, Luu TV, Lee NH, TLX1 Hawley RG . and NOTCH coregulate transcription in T cell acute lymphoblastic leukemia cells. Mol Cancer 2010; 9: 181.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 4th edn WHO Press, Geneva, Switzerland, 2008.
Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF et al. Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO J 1995; 14: 276–285.
Donehower LA . The p53-deficient mouse: a model for basic and applied cancer studies. Semin Cancer Biol 1996; 7: 269–278.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992; 356: 215–221.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994; 4: 1–7.
Imada K . Immunodeficient mouse models of lymphoid tumors. Int J Hematol 2003; 77: 336–341.
Asou H, Tashiro S, Hamamoto K, Otsuji A, Kita K, Kamada N . Establishment of a human acute myeloid leukemia cell line (Kasumi-1) with 8;21 chromosome translocation. Blood 1991; 77: 2031–2036.
Riccardi C, Nicoletti I . Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 2006; 1: 1458–1461.
Acknowledgements
The authors would like to thank Susanne Bösser for excellent technical support. This work was supported by grants from the German Cancer Aid Foundation (no. 108659; MZ), the German National Genome Research Network (NGFN project N1KR-S12T23, MZ) and the SFB ‘Lipotox’ of the Austrian Science foundation FWF (no. W30; GH). MZ, IMM and MAR are thankful for the support by the LOEWE Center for Cell and Gene Therapy Frankfurt (HMWK III L 4-518/17.004 (2010)). The Georg-Speyer-Haus is funded jointly by the German Federal Ministry of Health (BMG) and the Ministry of Higher Education, Research and the Arts of the state of Hessen (HMWK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Eißmann, M., Melzer, I., Fernández, S. et al. Overexpression of the anti-apoptotic protein AVEN contributes to increased malignancy in hematopoietic neoplasms. Oncogene 32, 2586–2591 (2013). https://doi.org/10.1038/onc.2012.263
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.263
Keywords
This article is cited by
-
Establishment and genomic characterization of gingivobuccal carcinoma cell lines with smokeless tobacco associated genetic alterations and oncogenic PIK3CA mutation
Scientific Reports (2019)
-
Heritability of in vitro phenotypes exhibited by murine adipose-derived stromal cells
Mammalian Genome (2016)