Abstract
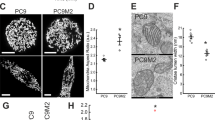
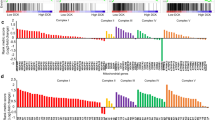
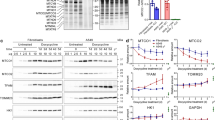
Primary mitochondrial dysfunction commonly leads to failure in cellular adaptation to stress. Paradoxically, however, nonsynonymous mutations of mitochondrial DNA (mtDNA) are frequently found in cancer cells and may have a causal role in the development of resistance to genotoxic stress induced by common chemotherapeutic agents, such as cis-diammine-dichloroplatinum(II) (cisplatin, CDDP). Little is known about how these mutations arise and the associated mechanisms leading to chemoresistance. Here, we show that the development of adaptive chemoresistance in the A549 non-small-cell lung cancer cell line to CDDP is associated with the hetero- to homoplasmic shift of a nonsynonymous mutation in MT-ND2, encoding the mitochondrial Complex-I subunit ND2. The mutation resulted in a 50% reduction of the NADH:ubiquinone oxidoreductase activity of the complex, which was compensated by increased biogenesis of respiratory chain complexes. The compensatory mitochondrial biogenesis was most likely mediated by the nuclear co-activators peroxisome proliferator-activated receptor gamma co-activator-1α (PGC-1α) and PGC-1β, both of which were significantly upregulated in the CDDP-resistant cells. Importantly, both transient and stable silencing of PGC-1β re-established the sensitivity of these cells to CDDP-induced apoptosis. Remarkably, the PGC-1β-mediated CDDP resistance was independent of the mitochondrial effects of the co-activator. Altogether, our results suggest that partial respiratory chain defects because of mtDNA mutations can lead to compensatory upregulation of nuclear transcriptional co-regulators, in turn mediating resistance to genotoxic stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lane N . Mitonuclear match: Optimizing fitness and fertility over generations drives ageing within generations. BioEssays 2011; 16: 1–10.
Duchen MR, Szabadkai G . Roles of mitochondria in human disease. Essays Biochem 2010; 47: 15–37.
Park CB, Larsson N-G, Mitochondrial DNA . Mutations in disease and aging. J Cell Biol 2011; 193: 809–818.
Spinazzola A, Zeviani M . Mitochondrial diseases: a cross-talk between mitochondrial and nuclear genomes. Adv Exp Med Biol 2009; 652: 69–84.
Kulawiec M, Salk JJ, Ericson NG, Wanagat J, Bielas JH . Generation, function, and prognostic utility of somatic mitochondrial DNA mutations in cancer. Environ Mol Mutagen 2010; 51: 427–439.
Chatterjee A, Mambo E, Sidransky D, Mitochondrial DNA . mutations in human cancer. Oncogene 2006; 25: 4663–4674.
Kwong JQ, Henning MS, Starkov AA, Manfredi G . The mitochondrial respiratory chain is a modulator of apoptosis. J Cell Biol 2007; 179: 1163–1177.
Wallace DC, Fan W . Energetics, epigenetics, mitochondrial genetics. Mitochondrion 2010; 10: 12–31.
Chandra D, Singh KK . Genetic insights into OXPHOS defect and its role in cancer. Biochem Biophys Acta 2011; 1807: 620–625.
Mizutani S, Miyato Y, Shidara Y, Asoh S, Tokunaga A, Tajiri T et al. Mutations in the mitochondrial genome confer resistance of cancer cells to anticancer drugs. Cancer Sci 2009; 100: 1680–1687.
Ohta S . Contribution of somatic mutations in the mitochondrial genome to the development of cancer and tolerance against anticancer drugs. Oncogene 2006; 25: 4768–4776.
Kulawiec M, Owens KM, Singh KK . Cancer cell mitochondria confer apoptosis resistance and promote metastasis. Cancer Biol Ther 2009; 8: 1378–1385.
Jones AWE, Yao Z, Vicencio JM, Karkucinska-Wieckowska A, Szabadkai G . PGC-1 family coactivators and cell fate: roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria-nucleus signalling. Mitochondrion 2012; 12: 86–99.
Lin J, Handschin C, Spiegelman BM . Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 2005; 1: 361–370.
Scarpulla RC . Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochem Biophys Acta 2011; 1813: 1269–1278.
Hock MB, Kralli A . Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 2009; 71: 177–203.
Butow RA, Avadhani NG . Mitochondrial signaling: the retrograde response. Mol Cell 2004; 14: 1–15.
Guerra F, Kurelac I, Cormio A, Zuntini R, Amato LB, Ceccarelli C et al. Placing mitochondrial DNA mutations within the progression model of type I endometrial carcinoma. Hum Mol Genet 2011; 20: 2394–2405.
Bianchi K, Vandecasteele G, Carli C, Romagnoli A, Szabadkai G, Rizzuto R . Regulation of Ca2+ signalling and Ca2+-mediated cell death by the transcriptional coactivator PGC-1alpha. Cell Death Differ 2006; 13: 586–596.
Rahman S, Ecob R, Costello H, Sweeney MG, Duncan AJ, Pearce K et al. Hearing in 44-45 year olds with m.1555A>G, a genetic mutation predisposing to aminoglycoside-induced deafness: a population based cohort study. BMJ open 2012; 2: e000411.
Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N . Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 1999; 23: 147.
Di Re M, Sembongi H, He J, Reyes a, Yasukawa T, Martinsson P et al. The accessory subunit of mitochondrial DNA polymerase gamma determines the DNA content of mitochondrial nucleoids in human cultured cells. Nucl Acid Res 2009; 37: 5701–5713.
Nakamaru-Ogiso E, Han H, Matsuno-Yagi A, Keinan E, Sinha SC, Yagi T et al. The ND2 subunit is labeled by a photoaffinity analogue of asimicin, a potent complex I inhibitor. FEBS Lett 2010; 584: 883–888.
Efremov RG, Baradaran R, Sazanov LA . The architecture of respiratory complex I. Nature 2010; 465: 441–445.
Crews S, Ojala D, Posakony J, Nishiguchi J, Attardi G . Nucleotide sequence of a region of human mitochondrial DNA containing the precisely identified origin of replication. Nature 1979; 277: 192–198.
Guha M, Fang J-K, Monks R, Birnbaum MJ, Avadhani NG . Activation of Akt is essential for the propagation of mitochondrial respiratory stress signaling and activation of the transcriptional coactivator heterogeneous ribonucleoprotein A2. Mol Biol Cell 2010; 21: 3578–3589.
Sen T, Sen N, Brait M, Begum S, Chatterjee A, Hoque MO et al. DeltaNp63alpha confers tumor cell resistance to cisplatin through the AKT1 transcriptional regulation. Cancer Res 2011; 71: 1167–1176.
Fernandez-Marcos PJ, Auwerx J . Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nut 2011; 93: 884S–890S.
Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev 2008; 22: 1397–1409.
Verger A, Quinlan KGR, Crofts LA, Spanò S, Corda D, Kable EPW et al. Mechanisms directing the nuclear localization of the CtBP family proteins. Mol Cell Biol 2006; 26: 4882–4894.
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006; 127: 397–408.
St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB et al. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem 2003; 278: 26597–26603.
Cullen KJ, Yang Z, Schumaker L, Guo Z . Mitochondria as a critical target of the chemotheraputic agent cisplatin in head and neck cancer. J Bioenerg Biomemebr 2007; 39: 43–50.
Huang H-L, Fang L-W, Lu S-P, Chou C-K, Luh T-Y, Lai M-Z . DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation. Oncogene 2003; 22: 8168–8177.
Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM . Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab 2006; 3: 333–341.
Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab 2010; 12: 633–642.
Galluzzi L, Zamzami N, de La Motte Rouge T, Lemaire C, Brenner C, Kroemer G . Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis 2007; 12: 803–813.
Ragan CI . Sub-fractionation of mitochondria and isolation of proteins of oxidative phosphorylation. In: Darley VM, Rickwood DWM (eds) Mitochondria: A Practical Approach. IRL Press, Oxford, 1988, pp 79–113.
Lo S, Tolner B, Taanman J-W, Cooper JM, Gu M, Hartley JA et al. Assessment of the significance of mitochondrial DNA damage by chemotherapeutic agents. Int j oncol 2005; 27: 337–344.
Traba J, Del Arco a, Duchen MR, Szabadkai G, Satrústegui J . SCaMC-1 promotes cancer cell survival by desensitizing mitochondrial permeability transition via ATP/ADP-mediated matrix Ca(2+) buffering. Cell Death Differ 2012; 19: 650–660.
Campanella M, Seraphim A, Abeti R, Casswell E, Echave P, Duchen MR . IF1, the endogenous regulator of the F(1)F(o)-ATPsynthase, defines mitochondrial volume fraction in HeLa cells by regulating autophagy. Biochem Biophys Acta 2009; 1787: 393–401.
Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR . Relation Between Mitochondrial Membrane Potential and ROS Formation. Methods mol biol 2012; 810: 183–205.
Ruhanen H, Borrie S, Szabadkai G, Tyynismaa H, Jones AWE, Kang D et al. Mitochondrial single-stranded DNA binding protein is required for maintenance of mitochondrial DNA and 7S DNA but is not required for mitochondrial nucleoid organisation. Biochem Biophys Acta 2010; 1803: 931–939.
Acknowledgements
We thank the excellent technical help of M Rahman (UCL Biosciences Molecular Biology Unit), K Pearce (UCL Genomics), the access to the Arrayscan VTI HCS Reader and the help of G Keen, M Elrayess and J Staddon from Eisai Europe Ltd; Professors M Duchen, C Boshoff and JM Funes for reagents and advice; JW Taanman for providing the A549 rho0 cell line, and D Housenloy for the Akt antibodies. The work was supported by Parkinson’s UK (G-0905) and the Medical Research Council (MRC-DTA) to GS, ZY and AJ MRW is supported by the Polish Ministry of Science and Higher Education under Grant NN407075137 and by the grant from the National Science Centre—decision number DEC-2011/01/M/NZ3/02128. JMS is a recipient of a PhD fellowship from the Foundation for Polish Science, EU, European Regional Development Fund and Operational Programme “Innovative economy”. ML is recipient of a fellowship from the Foundation for Polish Science (Programme Start) and the L’Oreal fellowship (For Women in Science). SR is supported by Great Ormond Street Hospital Children’s Charity. GK is supported by the European Union (ApoSys, ArtForce, ChemoRes) and the Ligue contre le Cancer (Laboratoire labellisé).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Yao, Z., Jones, A., Fassone, E. et al. PGC-1β mediates adaptive chemoresistance associated with mitochondrial DNA mutations. Oncogene 32, 2592–2600 (2013). https://doi.org/10.1038/onc.2012.259
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.259
Keywords
This article is cited by
-
The PGC-1/ERR network and its role in precision oncology
npj Precision Oncology (2019)
-
Systems biology of cisplatin resistance: past, present and future
Cell Death & Disease (2014)
-
The protein disulfide isomerases PDIA4 and PDIA6 mediate resistance to cisplatin-induced cell death in lung adenocarcinoma
Cell Death & Differentiation (2014)
-
The emergence of the mitochondrial genome as a partial regulator of nuclear function is providing new insights into the genetic mechanisms underlying age-related complex disease
Human Genetics (2014)