Abstract
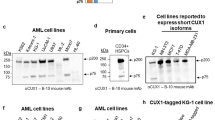
The RPS6KA6 gene encodes the p90 ribosomal S6 kinase-4 (RSK4) that is still largely uncharacterized. In this study we identified a new RSK4 transcription initiation site and several alternative splice sites with a 5′-RACE approach. The resulting mRNA variants encompass four possible first start codons. The first 15 nucleotides (nt) of exon 22 in mouse and the penultimate exon in both human (exon 21) and mouse (exon 24) RSK4 underwent alternative splicing, although the penultimate exon deleted variant appeared mainly in cell clines, but not in most normal tissues. Demethylation agent 5-azacytidine inhibited the deletion of the penultimate exon, whereas two indolocarbazole-derived inhibitors of cyclin-dependent kinase 4 or 6 induced deletion of the first 39 nt from exon 21 of human RSK4. In all human cancer cell lines studied, the 90-kDa wild-type RSK4 was sparse but, surprisingly, several isoforms at or smaller than 72 kDa were expressed as detected by seven different antibodies. On immunoblots, each of these smaller isoforms often appeared as a duplet or triplet and the levels of these isoforms varied greatly among different cell lines and culture conditions. Cyclin D1 inhibited RSK4 expression and serum starvation enhanced the inhibition, whereas c-Myc and RSK4 inhibited cyclin D1. The effects of RSK4 on cell growth, cell death and chemoresponse depended on the mRNA variant or the protein isoform expressed, on the specificity of the cell lines, as well as on the anchorage-dependent or -independent growth conditions and the in vivo situation. Moreover, we also observed that even a given cDNA might be expressed to multiple proteins; therefore, when using a cDNA, one needs to exclude this possibility before attribution of the biological results from the cDNA to the anticipated protein. Collectively, our results suggest that whether RSK4 is oncogenic or tumor suppressive depends on many factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Frodin M, Gammeltoft S . Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol 1999; 151: 65–77.
Hauge C, Frodin M . RSK and MSK in MAP kinase signalling. J Cell Sci 2006; 119: 3021–3023.
Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P . Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem 1998; 273: 1496–1505.
Anjum R, Blenis J . The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol 2008; 9: 747–758.
Kohn M, Hameister H, Vogel M, Kehrer-Sawatzki H . Expression pattern of the Rsk2, Rsk4 and Pdk1 genes during murine embryogenesis. Gene Expr Patterns 2003; 3: 173–177.
Bignone PA, Lee KY, Liu Y, Emilion G, Finch J, Soosay AE et alRPS6KA2 a putative tumour suppressor gene at 6q27 in sporadic epithelial ovarian cancer. Oncogene 2007; 26: 683–700.
Lin H, Morin PJ . A novel homozygous deletion at chromosomal band 6q27 in an ovarian cancer cell line delineates the position of a putative tumor suppressor gene. Cancer Lett 2001; 173: 63–70.
Yntema HG, van den HB, Kissing J, van DG, Poppelaars F, Chelly J et alA novel ribosomal S6-kinase (RSK4; RPS6KA6) is commonly deleted in patients with complex X-linked mental retardation. Genomics 1999; 62: 332–343.
Myers AP, Corson LB, Rossant J, Baker JC . Characterization of mouse Rsk4 as an inhibitor of fibroblast growth factor-RAS-extracellular signal-regulated kinase signaling. Mol Cell Biol 2004; 24: 4255–4266.
Dummler BA, Hauge C, Silber J, Yntema HG, Kruse LS, Kofoed B et alFunctional characterization of human RSK4, a new 90-kDa ribosomal S6 kinase, reveals constitutive activation in most cell types. J Biol Chem 2005; 280: 13304–13314.
Thakur A, Rahman KW, Wu J, Bollig A, Biliran H, Lin X et alAberrant expression of X-linked genes RbAp46, Rsk4, and Cldn2 in breast cancer. Mol Cancer Res 2007; 5: 171–181.
Niehof M, Borlak J . RSK4 and PAK5 are novel candidate genes in diabetic rat kidney and brain. Mol Pharmacol 2005; 67: 604–611.
Thakur A, Xu H, Wang Y, Bollig A, Biliran H, Liao JD . The role of X-linked genes in breast cancer. Breast Cancer Res Treat 2005; 93: 135–143.
Thakur A, Bollig A, Wu J, Liao DJ . Gene expression profiles in primary pancreatic tumors and metastatic lesions of Ela-c-myc transgenic mice. Mol Cancer 2008; 7 doi:10.1086/1476-4598-7-11.
Thakur A, Sun Y, Bollig A, Wu J, Biliran H, Banerjee S et alAnti-invasive and antimetastatic activities of ribosomal protein S6 kinase 4 in breast cancer cells. Clin Cancer Res 2008; 14: 4427–4436.
Dewdney SB, Rimel BJ, Thaker PH, Thompson DM, Schmidt A, Huettner P et alAberrant methylation of the X-linked ribosomal S6 kinase RPS6KA6 (RSK4) in endometrial cancers. Clin Cancer Res 2011; 17: 2120–2129.
Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M et alA large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 2004; 428: 431–437.
Lopez-Vicente L, Pons B, Coch L, Teixido C, Hernandez-Losa J, Armengol G et alRSK4 inhibition results in bypass of stress-induced and oncogene-induced senescence. Carcinogenesis 2011; 32: 470–476.
Lopez-Vicente L, Armengol G, Pons B, Coch L, Argelaguet E, Lleonart M et alRegulation of replicative and stress-induced senescence by RSK4, which is down-regulated in human tumors. Clin Cancer Res 2009; 15: 4546–4553.
LLeonart ME, Vidal F, Gallardo D, az-Fuertes M, Rojo F, Cuatrecasas M et alNew p53 related genes in human tumors: significant downregulation in colon and lung carcinomas. Oncol Rep 2006; 16: 603–608.
Bender C, Ullrich A . PRKX TTBK2 and RSK4 expression causes sunitinib resistance in kidney carcinoma- and melanoma cell lines. Int J Cancer 2011, e-pub ahead of print; doi:10.1002/ijc.26486.
Liao D, Thakur A, Wu J, Biliran H, Sarkar FH . Perspectives on c-Myc, Cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Crit Rev Oncog 2007; 13: 93–158.
Liao DJ, Dickson RB . c-Myc in breast cancer. Endocr Relat Cancer 2000; 7: 143–164.
Liao JD, Adsay NV, Khannani F, Grignon D, Thakur A, Sarkar FH . Histological complexities of pancreatic lesions from transgenic mouse models are consistent with biological and morphological heterogeneity of human pancreatic cancer. Histol Histopathol 2007; 22: 661–676.
Biliran H, Wang Y, Banerjee S, Xu H, Heng H, Thakur A et alOverexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line. Clin Cancer Res 2005; 11: 6075–6086.
Biliran H, Banerjee S, Thakur A, Sarkar FH, Bollig A, Ahmed F et alc-Myc-induced chemosensitization is mediated by suppression of cyclin D1 expression and nuclear factor-kappa B activity in pancreatic cancer cells. Clin Cancer Res 2007; 13: 2811–2821.
Yang M, Sun Y, Ma L, Wang C, Wu JM, Bi A et alComplex alternative splicing of the smarca2 gene suggests the importance of smarca2-B variants. J Cancer 2011; 2: 386–400.
Sun Y, Li YX, Wu HJ, Wu SH, Wang YA, Luo DZ et alEffects of an Indolocarbazole-derived CDK4 inhibitor on breast cancer cells. J Cancer 2011; 2: 36–51.
El-Kady A, Sun Y, Li YX, Liao DJ . Cyclin D1 inhibits whereas c-Myc enhances the cytotoxicity of cisplatin in mouse pancreatic cancer cells via regulation of several members of the NF-kappaB and Bcl-2 families. J Carcinog 2011; 10: 24.
Wang C, Lisanti MP, Liao DJ . Reviewing once more the c-myc and Ras collaboration: converging at the cyclin D1-CDK4 complex and challenging basic concepts of cancer biology. Cell Cycle 2011; 10: 57–67.
Fitzgerald KD, Semler BL . Bridging IRES elements in mRNAs to the eukaryotic translation apparatus. Biochem Biophys Acta 2009; 1789: 518–528.
Kozak M . Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene 2005; 361: 13–37.
Shatsky IN, Dmitriev SE, Terenin IM, Andreev DE . Cap- and IRES-independent scanning mechanism of translation initiation as an alternative to the concept of cellular IRESs. Mol Cells 2010; 30: 285–293.
Iacono M, Mignone F, Pesole G . uAUG and uORFs in human and rodent 5'untranslated mRNAs. Gene 2005; 349: 97–105.
Ivanov IP, Atkins JF, Michael AJ . A profusion of upstream open reading frame mechanisms in polyamine-responsive translational regulation. Nucleic Acids Res 2010; 38: 353–359.
Tanner DR, Cariello DA, Woolstenhulme CJ, Broadbent MA, Buskirk AR . Genetic identification of nascent peptides that induce ribosome stalling. J Biol Chem 2009; 284: 34809–34818.
Nyiko T, Sonkoly B, Merai Z, Benkovics AH, Silhavy D . Plant upstream ORFs can trigger nonsense-mediated mRNA decay in a size-dependent manner. Plant Mol Biol 2009; 71: 367–378.
Wethmar K, Smink JJ, Leutz A . Upstream open reading frames: molecular switches in (patho)physiology. Bioessays 2010; 32: 885–893.
Chatterjee S, Pal JK . Role of 5'- and 3'-untranslated regions of mRNAs in human diseases. Biol Cell 2009; 101: 251–262.
Le Quesne JP, Spriggs KA, Bushell M, Willis AE . Dysregulation of protein synthesis and disease. J Pathol 2010; 220: 140–151.
Kozak M . Faulty old ideas about translational regulation paved the way for current confusion about how microRNAs function. Gene 2008; 423: 108–115.
Kozak M . Lessons (not) learned from mistakes about translation. Gene 2007; 403: 194–203.
Kozak M . Some thoughts about translational regulation: forward and backward glances. J Cell Biochem 2007; 102: 280–290.
Kozak M . Rethinking some mechanisms invoked to explain translational regulation in eukaryotes. Gene 2006; 382: 1–11.
Kozak M . A second look at cellular mRNA sequences said to function as internal ribosome entry sites. Nucleic Acids Res 2005; 33: 6593–6602.
Vila-Perello M, Muir TW . Biological applications of protein splicing. Cell 2010; 143: 191–200.
Hinnebusch AG . Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 2005; 59: 407–450.
Szamecz B, Rutkai E, Cuchalova L, Munzarova V, Herrmannova A, Nielsen KH et aleIF3a cooperates with sequences 5' of uORF1 to promote resumption of scanning by post-termination ribosomes for reinitiation on GCN4 mRNA. Genes Dev 2008; 22: 2414–2425.
Calvo SE, Pagliarini DJ, Mootha VK . Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci USA 2009; 106: 7507–7512.
Arava Y, Boas FE, Brown PO, Herschlag D . Dissecting eukaryotic translation and its control by ribosome density mapping. Nucleic Acids Res 2005; 33: 2421–2432.
Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS . Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 2009; 324: 218–223.
Mercer TR, Wilhelm D, Dinger ME, Solda G, Korbie DJ, Glazov EA et alExpression of distinct RNAs from 3' untranslated regions. Nucleic Acids Res 2011; 39: 2393–2403.
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP . A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 2011; 146: 353–358.
Liao DZ, Pantazis CG, Hou X, Li SA . Promotion of estrogen-induced mammary gland carcinogenesis by androgen in the male Noble rat: probable mediation by steroid receptors. Carcinogenesis 1998; 19: 2173–2180.
Sumanasekera C, Watt DS, Stamm S . Substances that can change alternative splice-site selection. Biochem Soc Trans 2008; 36: 483–490.
Campbell MJ, Wollish WS, Lobo M, Esserman LJ . Epithelial and fibroblast cell lines derived from a spontaneous mammary carcinoma in a MMTV/neu transgenic mouse. In Vitro Cell Dev Biol Anim 2002; 38: 326–333.
Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM . Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res 1997; 57: 3325–3330.
Hinds PW, Dowdy SF, Eaton EN, Arnold A, Weinberg RA . Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci USA 1994; 91: 709–713.
Gresner P, Gromadzinska J, Wasowicz W . Reference genes for gene expression studies on non-small cell lung cancer. Acta Biochem Pol 2009; 56: 307–316.
Palazzo AF, Akef A . Nuclear export as a key arbiter of ‘mRNA identity’ in eukaryotes. Biochem Biophys Acta 2012; 1819: 566–577.
Sun Y, Sriramajayam K, Luo D, Liao DJ . A quick, cost-free method of purification of DNA fragments from agarose gel. J Cancer 2012; 3: 93–95.
Wang Y, Thakur A, Sun Y, Wu J, Biliran H, Bollig A et alSynergistic effect of cyclin D1 and c-Myc leads to more aggressive and invasive mammary tumors in severe combined immunodeficient mice. Cancer Res 2007; 67: 3698–3707.
Liao DZ, Hou X, Bai S, Li SA, Li JJ . Unusual deregulation of cell cycle components in early and frank estrogen-induced renal neoplasias in the Syrian hamster. Carcinogenesis 2000; 21: 2167–2173.
Cao S, McGuire JJ, Rustum YM . Antitumor activity of ZD1694 (tomudex) against human head and neck cancer in nude mouse models: role of dosing schedule and plasma thymidine. Clin Cancer Res 1999; 5: 1925–1934.
Acknowledgements
This work was supported by a NIH grant RO1 CA100864 and a Pardee Foundation grant on pancreatic cancer to DJ Liao. We would like to thank Dr Fred Bogott from Austin Medical Center at Austin of Minnesota for his excellent English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Sun, Y., Cao, S., Yang, M. et al. Basic anatomy and tumor biology of the RPS6KA6 gene that encodes the p90 ribosomal S6 kinase-4. Oncogene 32, 1794–1810 (2013). https://doi.org/10.1038/onc.2012.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.200
Keywords
This article is cited by
-
Effect of RSK4 on biological characteristics of colorectal cancer
World Journal of Surgical Oncology (2018)
-
Ribosomal s6 protein kinase 4: a prognostic factor for renal cell carcinoma
British Journal of Cancer (2013)