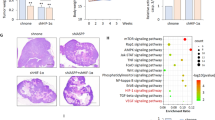
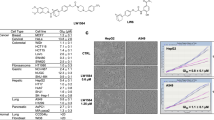
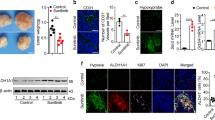
Abstract
Most solid tumors are characterized by a metabolic shift from glucose oxidation to glycolysis, in part due to actively suppressed mitochondrial function, a state that favors resistance to apoptosis. Suppressed mitochondrial function may also contribute to the activation of hypoxia-inducible factor 1α (HIF1α) and angiogenesis. We have previously shown that the inhibitor of pyruvate dehydrogenase kinase (PDK) dichloroacetate (DCA) activates glucose oxidation and induces apoptosis in cancer cells in vitro and in vivo. We hypothesized that DCA will also reverse the ‘pseudohypoxic’ mitochondrial signals that lead to HIF1α activation in cancer, even in the absence of hypoxia and inhibit cancer angiogenesis. We show that inhibition of PDKII inhibits HIF1α in cancer cells using several techniques, including HIF1α luciferase reporter assays. Using pharmacologic and molecular approaches that suppress the prolyl-hydroxylase (PHD)-mediated inhibition of HIF1α, we show that DCA inhibits HIF1α by both a PHD-dependent mechanism (that involves a DCA-induced increase in the production of mitochondria-derived α-ketoglutarate) and a PHD-independent mechanism, involving activation of p53 via mitochondrial-derived H2O2, as well as activation of GSK3β. Effective inhibition of HIF1α is shown by a decrease in the expression of several HIF1α regulated gene products as well as inhibition of angiogenesis in vitro in matrigel assays. More importantly, in rat xenotransplant models of non-small cell lung cancer and breast cancer, we show effective inhibition of angiogenesis and tumor perfusion in vivo, assessed by contrast-enhanced ultrasonography, nuclear imaging techniques and histology. This work suggests that mitochondria-targeting metabolic modulators that increase pyruvate dehydrogenase activity, in addition to the recently described pro-apoptotic and anti-proliferative effects, suppress angiogenesis as well, normalizing the pseudo-hypoxic signals that lead to normoxic HIF1α activation in solid tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gatenby RA, Gillies RJ . Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4: 891–899.
Michelakis ED, Webster L, Mackey JR . Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer 2008; 99: 989–994.
Pan JG, Mak TW . Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE 2007; 2007: pe14.
Vander Heiden MG, Cantley LC, Thompson CB . Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033.
Dromparis P, Sutendra G, Michelakis ED . The role of mitochondria in pulmonary vascular remodeling. J Mol Med 2010; 88: 1003–1010.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB . The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008; 7: 11–20.
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R et alA mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007; 11: 37–51.
Fantin VR, St-Pierre J, Leder P . Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006; 9: 425–434.
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R et alThe M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008; 452: 230–233.
Chen Y, Cairns R, Papandreou I, Koong A, Denko NC . Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS One 2009; 4: e7033.
Sun RC, Fadia M, Dahlstrom JE, Parish CR, Board PG, Blackburn AC . Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat 2010; 120: 253–260.
Sanchez-Arago M, Chamorro M, Cuezva JM . Selection of cancer cells with repressed mitochondria triggers colon cancer progression. Carcinogenesis 2010; 31: 567–576.
Cao W, Yacoub S, Shiverick KT, Namiki K, Sakai Y, Porvasnik S et alDichloroacetate (DCA) sensitizes both wild-type and over expressing Bcl-2 prostate cancer cells in vitro to radiation. Prostate 2008; 68: 1223–1231.
Wong JY, Huggins GS, Debidda M, Munshi NC, De Vivo I . Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecol Oncol 2008; 109: 394–402.
Saed GM, Fletcher NM, Jiang ZL, Abu-Soud HM, Diamond MP . Dichloroacetate induces apoptosis of epithelial ovarian cancer cells through a mechanism involving modulation of oxidative stress. Reprod Sci 2011; 18: 1253–1261.
Vella S, Conti M, Tasso R, Cancedda R, Pagano A . Dichloroacetate (DCA) inhibits neuroblastoma growth by specifically acting against malignant undifferentiated cells. Int J Cancer 2011; 130: 1484–1493.
Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E et alMetabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med 2010; 2: 31ra34.
Denko NC . Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008; 8: 705–713.
Semenza GL . Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE 2007; 2007: cm8.
Semenza GL . Hypoxia-inducible factors in physiology and medicine. Cell 2012; 148: 399–408.
McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND et alPyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem 2008; 283: 22700–22708.
Huang LE, Arany Z, Livingston DM, Bunn HF . Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem 1996; 271: 32253–32259.
Salceda S, Caro J . Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 1997; 272: 22642–22647.
Wang GL, Jiang BH, Semenza GL . Effect of altered redox states on expression and DNA-binding activity of hypoxia-inducible factor 1. Biochem Biophys Res Commun 1995; 212: 550–556.
Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M et alOxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 2005; 1: 409–414.
Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT et alMitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 2005; 1: 393–399.
MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM et alCell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol 2007; 27: 3282–3289.
Huang C, Zhang Z, Ding M, Li J, Ye J, Leonard SS et alVanadate induces p53 transactivation through hydrogen peroxide and causes apoptosis. J Biol Chem 2000; 275: 32516–32522.
Wang S, Leonard SS, Ye J, Ding M, Shi X . The role of hydroxyl radical as a messenger in Cr(VI)-induced p53 activation. Am J Physiol Cell Physiol 2000; 279: C868–C875.
Xie S, Wang Q, Wu H, Cogswell J, Lu L, Jhanwar-Uniyal M et alReactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J Biol Chem 2001; 276: 36194–36199.
Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS . Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J Biol Chem 2003; 278: 48872–48879.
Schmid T, Zhou J, Kohl R, Brune B . p300 relieves p53-evoked transcriptional repression of hypoxia-inducible factor-1 (HIF-1). Biochem J 2004; 380: 289–295.
Vousden KH, Ryan KM . p53 and metabolism. Nat Rev Cancer 2009; 9: 691–700.
Kaluzova M, Kaluz S, Lerman MI, Stanbridge EJ . DNA damage is a prerequisite for p53-mediated proteasomal degradation of HIF-1alpha in hypoxic cells and downregulation of the hypoxia marker carbonic anhydrase IX. Mol Cell Biol 2004; 24: 5757–5766.
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q et alRegulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev 2000; 14: 34–44.
Papandreou I, Goliasova T, Denko NC . Anti-cancer drugs that target metabolism, is dichloroacetate the new paradigm? Int J Cancer 2011; 128: 1001–1008.
Muangnoi P, Lu M, Lee J, Thepouyporn A, Mirzayans R, Le XC et alCytotoxicity, apoptosis and DNA damage induced by Alpinia galanga rhizome extract. Planta Med 2007; 73: 748–754.
Zamzami N, Kroemer G . The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol 2001; 2: 67–71.
Chen LB . Mitochondrial membrane potential in living cells. Annu Rev Cell Biol 1988; 4: 155–181.
Chandel NS, Vander Heiden MG, Thompson CB, Schumacker PT . Redox regulation of p53 during hypoxia. Oncogene 2000; 19: 3840–3848.
Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S et alCell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 2003; 93: 1074–1081.
Okuyama H, Krishnamachary B, Zhou YF, Nagasawa H, Bosch-Marce M, Semenza GL . Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem 2006; 281: 15554–15563.
Chan DA, Sutphin PD, Denko NC, Giaccia AJ . Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1alpha. J Biol Chem 2002; 277: 40112–40117.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR et alC. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001; 107: 43–54.
Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V et alHIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 2007; 12: 230–238.
Pastorino JG, Hoek JB, Hexokinase II . the integration of energy metabolism and control of apoptosis. Curr Med Chem 2003; 10: 1535–1551.
Pastorino JG, Hoek JB, Shulga N . Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res 2005; 65: 10545–10554.
Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD et alFatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med 2010; 2: 44ra58.
Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M et alRegulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem 2003; 278: 31277–31285.
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD et alActivation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996; 16: 4604–4613.
Pugh CW, Ratcliffe PJ . Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 2003; 9: 677–684.
Karshovska E, Zernecke A, Sevilmis G, Millet A, Hristov M, Cohen CD et alExpression of HIF-1alpha in injured arteries controls SDF-1alpha mediated neointima formation in apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol 2007; 27: 2540–2547.
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME et alProgenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004; 10: 858–864.
Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA . Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res 2006; 66: 9054–9064.
Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B et alMesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 2009; 4: e4992.
Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z et alPDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 2009; 15: 21–34.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et alMinimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317.
Colter DC, Class R, DiGirolamo CM, Prockop DJ . Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA 2000; 97: 3213–3218.
Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F et alDissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008; 233: 901–913.
Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE . Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2006; 24: 1030–1041.
Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M et alEffect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003; 362: 697–703.
Sutendra G, Dromparis P, Wright P, Bonnet S, Haromy A, Hao Z et alThe role of nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med 2011; 3: 88ra55.
Douwes DPB, Hogendoorn PC, Kuipers-Dijkshoorn N, Prins FA, van Duinen SG, Taschner PE et alSDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. J Pathol 2003; 201: 480–486.
Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D et alGermline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 2002; 30: 406–410.
Sutendra G, Dromparis P, Bonnet S, Haromy A, McMurtry MS, Bleackley RC et alPyruvate dehydrogenase inhibition by the inflammatory cytokine TNFalpha contributes to the pathogenesis of pulmonary arterial hypertension. J Mol Med 2011; 89: 771–783.
Semenza GL . Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–732.
Bhattacharyya A, Chattopadhyay R, Hall EH, Mebrahtu ST, Ernst PB, Crowe SE . Mechanism of hypoxia-inducible factor 1 alpha-mediated Mcl1 regulation in Helicobacter pylori-infected human gastric epithelium. Am J Physiol Gastrointest Liver Physiol 2010; 299: G1177–G1186.
Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ . Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem 2008; 283: 28106–28114.
Kim JW, Tchernyshyov I, Semenza GL, Dang CV . HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 2006; 3: 177–185.
Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R et alPyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 2011; 145: 732–744.
Francia G, Emmenegger U, Kerbel RS . Tumor-associated fibroblasts as ‘Trojan Horse’ mediators of resistance to anti-VEGF therapy. Cancer Cell 2009; 15: 3–5.
Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM . Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J 1998; 329 (Pt 1): 191–196.
Knoechel TR, Tucker AD, Robinson CM, Phillips C, Taylor W, Bungay PJ et alRegulatory roles of the N-terminal domain based on crystal structures of human pyruvate dehydrogenase kinase 2 containing physiological and synthetic ligands. Biochemistry 2006; 45: 402–415.
Li J, Kato M, Chuang DT . Pivotal role of the C-terminal DW-motif in mediating inhibition of pyruvate dehydrogenase kinase 2 by dichloroacetate. J Biol Chem 2009; 284: 34458–34467.
Babu E, Ramachandran S, Coothankandaswamy V, Elangovan S, Prasad PD, Ganapathy V et alRole of SLC5A8, a plasma membrane transporter and a tumor suppressor, in the antitumor activity of dichloroacetate. Oncogene 2011; 30: 4026–4037.
Acknowledgements
This study was funded by grants from the Canadian Institutes for Health Research (CIHR) and Alberta Innovates Health Solutions (AIHS) to EDM. We would like to thank Dr Gregg Semenza for his help, providing materials and advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Sutendra, G., Dromparis, P., Kinnaird, A. et al. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene 32, 1638–1650 (2013). https://doi.org/10.1038/onc.2012.198
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.198
Keywords
This article is cited by
-
The expression pattern of pyruvate dehydrogenase kinases predicts prognosis and correlates with immune exhaustion in clear cell renal cell carcinoma
Scientific Reports (2023)
-
PI3K signalling at the intersection of cardio-oncology networks: cardiac safety in the era of AI
Cellular and Molecular Life Sciences (2022)
-
Extracellular vesicles derived from Lactobacillus plantarum restore chemosensitivity through the PDK2-mediated glucose metabolic pathway in 5-FU-resistant colorectal cancer cells
Journal of Microbiology (2022)
-
Dichloroacetate and PX-478 exhibit strong synergistic effects in a various number of cancer cell lines
BMC Cancer (2021)
-
Nitric oxide-releasing micelles with intelligent targeting for enhanced anti-tumor effect of cisplatin in hypoxia
Journal of Nanobiotechnology (2021)