Abstract
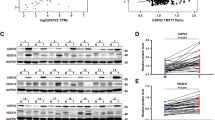
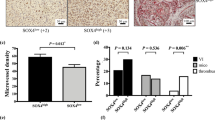
Hepatocellular carcinoma (HCC) typically relies on angiogenesis for its malignant behavior, including growth and metastasis. Vasohibin 2 (VASH2) was previously identified as an angiogenic factor, but its role in tumorigenesis is unknown. Using quantitative PCR and western blot analyses, we found that VASH2 is overexpressed in HCC cells and tissues. Using chromatin immunoprecipitation, we detected histone modifications at the putative VASH2 promoter, with increased H3K4 trimethylation and H3 acetylation and decreased H3K27 trimethylation, suggesting that epigenetic mechanisms are responsible for the deregulated VASH2 transcription in HCC. Knockdown of VASH2 via siRNA inhibited the proliferation of the hepatoma cell lines by delaying cell cycle progression and increasing apoptosis. Importantly, we found VASH2 secreted in the culture supernatant, and co-expression of its secretory chaperone small vasohibin-binding protein (SVBP) further enhanced VASH2 secretion. The supernatant from HepG2 cells expressing VASH2 enhanced the proliferation, migration and tube formation of human umbilical vein endothelial cells, and knockdown of VASH2 significantly inhibited these effects. In an in vivo study using a nude mouse model, we found that exogenous VASH2 significantly contributed to tumor growth, microvessel density and hemoglobin concentration in the tumors. Further analyses showed that the VASH2-mediated increase in the transcription of fibroblast growth factor-2, vascular endothelial growth factor and vasohibin 1 may be the mechanism underlying these effects. Taken together, these data indicate that VASH2 is abnormally expressed in HCC cells as a result of histone modifications and that VASH2 contributes to the angiogenesis in HCC via an SVBP-mediated paracrine mechanism. These results indicate a novel and important role for VASH2 in HCC angiogenesis and malignant transformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Semela D, Dufour JF . Angiogenesis and hepatocellular carcinoma. J Hepatol 2004; 41: 864–880.
Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP . High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res 2000; 6: 1900–1908.
Doger FK, Meteoglu I, Tuncyurek P, Okyay P, Cevikel H . Does the EGFR and VEGF expression predict the prognosis in colon cancer? Eur Surg Res 2006; 38: 540–544.
Cha HJ, Lee HH, Chae SW, Cho WJ, Kim YM, Choi HJ et alTristetraprolin downregulates the expression of both VEGF and COX-2 in human colon cancer. Hepatogastroenterology 2011; 58: 790–795.
Folkman J . Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007; 6: 273–286.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF et alSorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390.
Zhu AX, Duda DG, Sahani DV, Jain RK . HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol 2011; 8: 292–301.
Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M et alVasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest 2004; 114: 898–907.
Yoshinaga K, Ito K, Moriya T, Nagase S, Takano T, Niikura H et alRoles of intrinsic angiogenesis inhibitor, vasohibin, in cervical carcinomas. Cancer Sci 2011; 102: 446–451.
Tamaki K, Moriya T, Sato Y, Ishida T, Maruo Y, Yoshinaga K et alVasohibin-1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci 2009; 100: 88–94.
Shibuya T, Watanabe K, Yamashita H, Shimizu K, Miyashita H, Abe M et alIsolation and characterization of vasohibin-2 as a homologue of VEGF-inducible endothelium-derived angiogenesis inhibitor vasohibin. Arterioscler Thromb Vasc Biol 2006; 26: 1051–1057.
Kimura H, Miyashita H, Suzuki Y, Kobayashi M, Watanabe K, Sonoda H et alDistinctive localization and opposed roles of vasohibin-1 and vasohibin-2 in the regulation of angiogenesis. Blood 2009; 113: 4810–4818.
Strahl BD, Allis CD . The language of covalent histone modifications. Nature 2000; 403: 41–45.
Jenuwein T, Allis CD . Translating the histone code. Science 2001; 293: 1074–1080.
Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC et alActive genes are tri-methylated at K4 of histone H3. Nature 2002; 419: 407–411.
Plath K, Fang J, Mlynarczyk-Evans SK, Cao R, Worringer KA, Wang H et alRole of histone H3 lysine 27 methylation in X inactivation. Science 2003; 300: 131–135.
Gorisch SM, Wachsmuth M, Toth KF, Lichter P, Rippe K . Histone acetylation increases chromatin accessibility. J Cell Sci 2005; 118 (Part 24): 5825–5834.
Suzuki Y, Kobayashi M, Miyashita H, Ohta H, Sonoda H, Sato Y . Isolation of a small vasohibin-binding protein (SVBP) and its role in vasohibin secretion. J Cell Sci 2010; 123 (Part 18): 3094–3101.
Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V et alTNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 2010; 7: 455–469.
Tammali R, Reddy AB, Srivastava SK, Ramana KV . Inhibition of aldose reductase prevents angiogenesis in vitro and in vivo. Angiogenesis 2011; 14: 209–221.
Mi J, Zhang X, Liu Y, Reddy SK, Rabbani ZN, Sullenger BA et alNF-kappaB inhibition by an adenovirus expressed aptamer sensitizes TNFalpha-induced apoptosis. Biochem Biophys Res Commun 2007; 359: 475–480.
Yang ZF, Poon RT . Vascular changes in hepatocellular carcinoma. Anat Rec (Hoboken) 2008; 291: 721–734.
Wu XZ, Xie GR, Chen D . Hypoxia and hepatocellular carcinoma: the therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol 2007; 22: 1178–1182.
Jain RK, Duda DG, Clark JW, Loeffler JS . Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol 2006; 3: 24–40.
Sitohy B, Nagy JA, Jaminet SC, Dvorak HF . Tumor surrogate blood vessel subtypes exhibit differential susceptibility to anti-VEGF therapy. Cancer Res 2011; 71: 7021–7028.
Wang F, Xue X, Wei J, An Y, Yao J, Cai H et alhsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer 2010; 103: 567–574.
Eccles SA, Court W, Patterson L, Sanderson S . In vitro assays for endothelial cell functions related to angiogenesis: proliferation, motility, tubular differentiation, and proteolysis. Method Mol Biol 2009; 467: 159–181.
Liu LZ, Fang J, Zhou Q, Hu X, Shi X, Jiang BH . Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: implication of chemoprevention of lung cancer. Mol Pharmacol 2005; 68: 635–643.
Weidner N . Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 1995; 36: 169–180.
Acknowledgements
We are very grateful to Professor Yujie Sun from Nanjing Medical University for continuous technical support. This work was supported by grants from the National Nature Science Foundation of China (no. 81172267 and 30901627).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Xue, X., Gao, W., Sun, B. et al. Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene 32, 1724–1734 (2013). https://doi.org/10.1038/onc.2012.177
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.177
Keywords
This article is cited by
-
Comparison of gene expression between human and mouse iPSC-derived cardiomyocytes for stem cell therapies of cardiovascular defects via bioinformatic analysis
Translational Medicine Communications (2023)
-
Endoplasmic reticulum resident oxidase ERO1-Lalpha promotes hepatocellular carcinoma metastasis and angiogenesis through the S1PR1/STAT3/VEGF-A pathway
Cell Death & Disease (2018)
-
Vasohibin 2 reduces chemosensitivity to gemcitabine in pancreatic cancer cells via Jun proto-oncogene dependent transactivation of ribonucleotide reductase regulatory subunit M2
Molecular Cancer (2017)
-
RETRACTED ARTICLE: miR-1301 inhibits hepatocellular carcinoma cell migration, invasion, and angiogenesis by decreasing Wnt/β-catenin signaling through targeting BCL9
Cell Death & Disease (2017)
-
SOX17 increases the cisplatin sensitivity of an endometrial cancer cell line
Cancer Cell International (2016)