Abstract
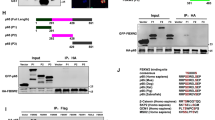
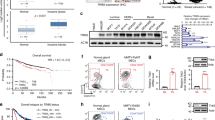
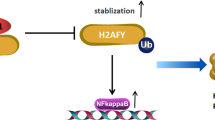
Human basal-like breast cancer (BLBC) is an enigmatic and aggressive malignancy with a poor prognosis. There is an urgent need to identify therapeutic targets for BLBC, because current treatment modalities are limited and not effective. The forkhead box transcription factor FOXC1 has recently been identified as a critical functional biomarker for BLBC. However, how it orchestrates BLBC cells was not clear. Here we show that FOXC1 activates the transcription factor nuclear factor-κB (NF-κB) in BLBC cells by increasing p65/RelA protein stability. High NF-κB activity has been associated with estrogen receptor-negative breast cancer, particularly BLBC. The effect of FOXC1 on p65/RelA protein stability is mediated by increased expression of Pin1, a peptidyl-prolyl isomerase. FOXC1 requires NF-κB for its regulation of cell proliferation, migration and invasion. Notably, FOXC1 overexpression renders breast cancer cells more susceptible to pharmacological inhibition of NF-κB. These results suggest that BLBC cells may rely on FOXC1-driven NF-κB signaling. Interventions of this pathway may provide modalities for the treatment of BLBC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dairkee SH, Puett L, Hackett AJ . Expression of basal and luminal epithelium-specific keratins in normal, benign, and malignant breast tissue. J Natl Cancer Inst 1988; 80: 691–695.
Wetzels RHW, Holland R, Haelst UJGMv, Lane EB, Leigh IM, Ramaekers FCS . Detection of basement membrane components and basal cell keratin 14 in noninvasive and invasive carcinomas of the breast. Am J Pathol 1989; 134: 571–579.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al. Molecular portraits of human breast tumours. Nature 2000; 406: 747–752.
Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res 2007; 9: R65.
Rakha EA, Reis-Filho JS, Ellis IO . Basal-like breast cancer: a critical review. J Clin Oncol 2008; 26: 2568–2581.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006; 295: 2492–2502.
Seewaldt VL, Scott V . Images in clinical medicine. Rapid progression of basal-type breast cancer. N Engl J Med 2007; 356: e12.
Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol 2006; 19: 264–271.
Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J . Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 2008; 68: 989–997.
Collett K, Stefansson IM, Eide J, Braaten A, Wang H, Eide GE et al. A basal epithelial phenotype is more frequent in interval breast cancers compared with screen detected tumors. Cancer Epidemiol Biomarkers Prev 2005; 14: 1108–1112.
Dejeux E, Ronneberg JA, Solvang H, Bukholm I, Geisler S, Aas T et al. DNA methylation profiling in doxorubicin treated primary locally advanced breast tumours identifies novel genes associated with survival and treatment response. Mol Cancer 2010; 9: 68.
Ray PS, Wang J, Qu Y, Sim MS, Shamonki J, Bagaria SP et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res 2010; 70: 3870–3876.
Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A 2010; 107: 15449–15454.
Nishimura DY, Swiderski RE, Alward WL, Searby CC, Patil SR, Bennet SR et al. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat Genet 1998; 19: 140–147.
Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, Hogan BL . The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell 1998; 93: 985–996.
Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, Parlee M et al. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. Am J Hum Genet 1998; 63: 1316–1328.
Kidson SH, Kume T, Deng K, Winfrey V, Hogan BL . The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Dev Biol 1999; 211: 306–322.
Smith RS, Zabaleta A, Kume T, Savinova OV, Kidson SH, Martin JE et al. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet 2000; 9: 1021–1032.
Moulder SL . Does the PI3K pathway play a role in basal breast cancer? Clin Breast Cancer 2010; 10: S66–S71.
Rexer BN, Ghosh R, Arteaga CL . Inhibition of PI3K and MEK: it is all about combinations and biomarkers. Clin Cancer Res 2009; 15: 4518–4520.
Lu S, Simin K, Khan A, Mercurio AM . Analysis of integrin beta4 expression in human breast cancer: association with basal-like tumors and prognostic significance. Clin Cancer Res 2008; 14: 1050–1058.
Lee CW, Simin K, Liu Q, Plescia J, Guha M, Khan A et al. A functional Notch-survivin gene signature in basal breast cancer. Breast Cancer Res 2008; 10: R97.
Yamaguchi N, Ito T, Azuma S, Ito E, Honma R, Yanagisawa Y et al. Constitutive activation of nuclear factor-kappaB is preferentially involved in the proliferation of basal-like subtype breast cancer cell lines. Cancer Sci 2009; 100: 1668–1674.
Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet Jr RJ, Sledge Jr GW . Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol 1997; 17: 3629–3639.
Biswas DK, Iglehart JD . Linkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancer. J Cell Physiol 2006; 209: 645–652.
Gershtein ES, Scherbakov AM, Platova AM, Tchemeris GY, Letyagin VP, Kushlinskii NE . The expression and DNA-binding activity of NF-kappaB nuclear transcription factor in the tumors of patients with breast cancer. Bull Exp Biol Med 2010; 150: 71–74.
Peng SL . Interplay between the NF-kappaB and forkhead transcription factors. Cell Death Differ 2005; 12: 699–701.
Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G et al. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell 2003; 12: 1413–1426.
Lu KP . Prolyl isomerase Pin1 as a molecular target for cancer diagnostics and therapeutics. Cancer Cell 2003; 4: 175–180.
Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Petkova V et al. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. Embo J 2001; 20: 3459–3472.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006; 10: 515–527.
Hoeflich KP, O’Brien C, Boyd Z, Cavet G, Guerrero S, Jung K et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res 2009; 15: 4649–4664.
Acknowledgements
We thank Yixian Zheng for the HA-ubiquitin plasmid, Fred Miller for 4T1 breast cancer cells, Inder M Verma for IKK double-knockout MEFs, Amer A Beg for p65-null MEFs, and Dave Hoon and Myles Cabot for technical support. This work was supported by National Institutes of Health (CA151610), QVC and the Fashion Footwear Association of New York Charitable Foundation, Associates for Breast & Prostate Cancer Studies, and the Avon Foundation (02-2010-068) to XC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Wang, Dr Ray, Dr Bagaria and Dr Cui are named inventors on patent applications regarding the role of FOXC1 in cancer.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, J., Ray, P., Sim, MS. et al. FOXC1 regulates the functions of human basal-like breast cancer cells by activating NF-κB signaling. Oncogene 31, 4798–4802 (2012). https://doi.org/10.1038/onc.2011.635
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.635
Keywords
This article is cited by
-
Reciprocal regulation of forkhead box C1 and L1 cell adhesion molecule contributes to triple-negative breast cancer progression
Breast Cancer Research and Treatment (2024)
-
Identification of hub genes to determine drug-disease correlation in breast carcinomas
Medical Oncology (2023)
-
Molecular characterization of Richter syndrome identifies de novo diffuse large B-cell lymphomas with poor prognosis
Nature Communications (2023)
-
Overexpression of FOXC1 Promotes Tumor Metastasis by Activating the Wnt/β-Catenin Signaling Pathway in Gastric Cancer
Digestive Diseases and Sciences (2022)
-
NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer
Journal of Biomedical Science (2021)