Abstract
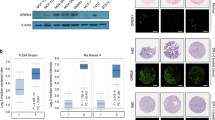
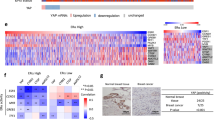
Insulin-like growth factor II (IGF-II) mRNA-binding protein 3 (IMP3) is emerging as a useful indicator of the progression and outcome of several cancers. IMP3 expression is associated with triple-negative breast carcinomas (TNBCs), which are aggressive tumors associated with poor outcome. In this study, we addressed the hypothesis that signaling pathways, which are characteristic of TNBCs, impact the expression of IMP3 and that IMP3 contributes to the function of TNBCs. The data obtained reveal that IMP3 expression is repressed specifically by estrogen receptor β (ERβ) and its ligand 3βA-diol but not by ERα. Epidermal growth factor receptor (EGFR) signaling and consequent activation of the mitogen-activated protein kinase pathway induce IMP3 transcription and expression. Interestingly, we discovered that the EGFR promoter contains an imperfect estrogen response element and that ERβ represses EGFR transcription. These data support a mechanism in which ERβ inhibits IMP3 expression indirectly by repressing the EGFR. This mechanism relates to the biology of TNBC, which is characterized by diminished ERβ and increased EGFR expression. We also demonstrate that IMP3 contributes to the migration and invasion of breast carcinoma cells. Given that IMP3 is an mRNA-binding protein, we determined that it binds several key mRNAs that could contribute to migration and invasion, including CD164 (endolyn) and MMP9. Moreover, expression of these mRNAs is repressed by ERβ and enhanced by EGFR signaling, consistent with our proposed mechanism for the regulation of IMP3 expression in breast cancer cells. Our findings show that IMP3 is an effector of EGFR-mediated migration and invasion and they provide the first indication of how this important mRNA-binding protein is regulated in cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC . A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol 1999; 19: 1262–1270.
Mueller-Pillasch F, Pohl B, Wilda M, Lacher U, Beil M, Wallrapp C et al. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech Develop 1999; 88: 95–99.
Mueller-Pillasch F, Lacher U, Wallrapp C, Micha A, Zimmerhackl F, Hameister H et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 1997; 14: 2729–2733.
Jiang Z, Chu PG, Woda BA, Rock KL, Liu Q, Hsieh CC et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol 2006; 7: 556–564.
Li C, Rock KL, Woda BA, Jiang Z, Fraire AE, Dresser K . IMP3 is a novel biomarker for adenocarcinoma in situ of the uterine cervix: an immunohistochemical study in comparison with p16(INK4a) expression. Modern Pathol 2007; 20: 242–247.
Lu D, Yang X, Jiang NY, Woda BA, Liu Q, Dresser K et al. IMP3, a new biomarker to predict progression of cervical intraepithelial neoplasia into invasive cancer. Am J Surg Pathol 2011; 35: 1638–1645.
Simon R, Bourne PA, Yang Q, Spaulding BO, di Sant’Agnese PA, Wang HL et al. Extrapulmonary small cell carcinomas express K homology domain containing protein overexpressed in cancer, but carcinoid tumors do not. Hum Pathol 2007; 38: 1178–1183.
Yantiss RK, Woda BA, Fanger GR, Kalos M, Whalen GF, Tada H et al. KOC (K homology domain containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol 2005; 29: 188–195.
Zheng W, Yi X, Fadare O, Liang SX, Martel M, Schwartz PE et al. The oncofetal protein IMP3: a novel biomarker for endometrial serous carcinoma. Am J Surg Pathol 2008; 32: 304–315.
Walter O, Prasad M, Lu S, Quinlan RM, Edmiston KL, Khan A . IMP3 is a novel biomarker for triple negative invasive mammary carcinoma associated with a more aggressive phenotype. Hum Pathol 2009; 40: 1528–1533.
Li S, Cha J, Kim J, Kim KY, Kim HJ, Nam W et al. Insulin-like growth factor II mRNA-binding protein 3: a novel prognostic biomarker for oral squamous cell carcinoma. Head Neck 2011; 33: 368–374.
Li D, Yan D, Tang H, Zhou C, Fan J, Li S et al. IMP3 is a novel prognostic marker that correlates with colon cancer progression and pathogenesis. Ann Surg Oncol 2009; 16: 3499–3506.
Irvin Jr WJ, Carey LA . What is triple-negative breast cancer? Eur J Cancer 2008; 44: 2799–2805.
Marotti JD, Collins LC, Hu R, Tamimi RM . Estrogen receptor-beta expression in invasive breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Modern Pathol 2010; 23: 197–204.
Foley J, Nickerson NK, Nam S, Allen KT, Gilmore JL, Nephew KP et al. EGFR signaling in breast cancer: bad to the bone. Semin Cell Dev Biol 2010; 21: 951–960.
Guerini V, Sau D, Scaccianoce E, Rusmini P, Ciana P, Maggi A et al. The androgen derivative 5alpha-androstane-3beta,17beta-diol inhibits prostate cancer cell migration through activation of the estrogen receptor beta subtype. Cancer Res 2005; 65: 5445–5453.
Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, Wang ZY . A positive feedback loop of ER-alpha36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene 2011; 30: 770–780.
Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW . Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 2003; 284: 31–53.
Klinge CM . Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 2001; 29: 2905–2919.
Klein-Hitpass L, Schorpp M, Wagner U, Ryffel GU . An estrogen-responsive element derived from the 5′ flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell 1986; 46: 1053–1061.
Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 2010; 141: 129–141.
Havens AM, Jung Y, Sun YX, Wang J, Shah RB, Buhring HJ et al. The role of sialomucin CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC Cancer 2006; 6: 195.
Ellenbroek SI, Collard JG . Rho GTPases: functions and association with cancer. Clin Exp Metastas 2007; 24: 657–672.
Reimer CL, Borras AM, Kurdistani SK, Garreau JR, Chung M, Aaronson SA et al. Altered regulation of cyclin G in human breast cancer and its specific localization at replication foci in response to DNA damage in p53+/+ cells. J Biol Chem 1999; 274: 11022–11029.
Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996; 384: 470–474.
Vikesaa J, Hansen TV, Jonson L, Borup R, Wewer UM, Christiansen J et al. RNA-binding IMPs promote cell adhesion and invadopodia formation. EMBO J 2006; 25: 1456–1468.
Yu Q, Stamenkovic I . Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Gene Dev 1999; 13: 35–48.
van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 2002; 161: 1991–1996.
Korsching E, Jeffrey SS, Meinerz W, Decker T, Boecker W, Buerger H . Basal carcinoma of the breast revisited: an old entity with new interpretations. J Clin Pathol 2008; 61: 553–560.
Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC . Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 2004; 64: 423–428.
Webb P, Valentine C, Nguyen P, Price Jr RH, Marimuthu A, West BL et al. ERbeta binds N-CoR in the presence of estrogens via an LXXLL-like motif in the N-CoR C-terminus. Nuclear Receptor 2003; 1: 4.
Pinton G, Thomas W, Bellini P, Manente AG, Favoni RE, Harvey BJ et al. Estrogen receptor beta exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib. PloS One 2010; 5: e14110.
Ryden L, Jirstrom K, Haglund M, Stal O, Ferno M . Epidermal growth factor receptor and vascular endothelial growth factor receptor 2 are specific biomarkers in triple-negative breast cancer. Results from a controlled randomized trial with long-term follow-up. Breast Cancer Res Tr 2010; 120: 491–498.
Garcia Castro C, Ravina M, Castro V, Salido EC . Expression of epidermal growth factor receptor (proto-oncogene c-erbB-1) and estrogen receptor in human breast carcinoma. An immunocytochemical study of 70 cases. Arch Gynecol Obstet 1993; 252: 169–177.
Martinazzi M, Crivelli F, Zampatti C, Martinazzi S . Epidermal growth factor receptor immunohistochemistry in different histological types of infiltrating breast carcinoma. J Clin Pathol 1993; 46: 1009–1010.
Newby JC, A’Hern RP, Leek RD, Smith IE, Harris AL, Dowsett M . Immunohistochemical assay for epidermal growth factor receptor on paraffin-embedded sections: validation against ligand-binding assay and clinical relevance in breast cancer. Brit J Cancer 1995; 71: 1237–1242.
Heldin CH, Westermark B . Growth factors: mechanism of action and relation to oncogenes. Cell 1984; 37: 9–20.
Xu L, Nilsson MB, Saintigny P, Cascone T, Herynk MH, Du Z et al. Epidermal growth factor receptor regulates MET levels and invasiveness through hypoxia-inducible factor-1alpha in non-small cell lung cancer cells. Oncogene 2010; 29: 2616–2627.
Van Meter TE, Broaddus WC, Rooprai HK, Pilkington GJ, Fillmore HL . Induction of membrane-type-1 matrix metalloproteinase by epidermal growth factor-mediated signaling in gliomas. Neuro-Oncology 2004; 6: 188–199.
Li Z, Xu X, Bai L, Chen W, Lin Y . Epidermal growth factor receptor-mediated tissue transglutaminase overexpression couples acquired tumor necrosis factor-related apoptosis-inducing ligand resistance and migration through c-FLIP and MMP-9 proteins in lung cancer cells. J Biol Chem 2011; 286: 21164–21172.
Agrawal A, Gutteridge E, Gee JM, Nicholson RI, Robertson JF . Overview of tyrosine kinase inhibitors in clinical breast cancer. Endocr-Relat Cancer 2005; 12 (Suppl 1): S135–S144.
Suvasini R, Shruti B, Thota B, Shinde SV, Friedmann-Morvinski D, Nawaz Z et al. Insulin growth factor-2 binding protein 3 (IGF2BP3) is a glioblastoma-specific marker that activates phosphatidylinositol 3-kinase/mitogen-activated protein kinase (PI3K/MAPK) pathways by modulating IGF-2. J Biol Chem 2011; 286: 25882–25890.
Liao B, Hu Y, Brewer G . RNA-binding protein insulin-like growth factor mRNA-binding protein 3 (IMP-3) promotes cell survival via insulin-like growth factor II signaling after ionizing radiation. J Biol Chem 2011; 286: 31145–31152.
Zeng ZS, Cohen AM, Guillem JG . Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis 1999; 20: 749–755.
Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G et al. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res 2003; 63: 5224–5229.
Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T et al. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Brit J Cancer 2001; 84: 1488–1496.
Liao B, Hu Y, Herrick DJ, Brewer G . The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J Biol Chem 2005; 280: 18517–18524.
Ellis MJ, Jenkins S, Hanfelt J, Redington ME, Taylor M, Leek R et al. Insulin-like growth factors in human breast cancer. Breast Cancer Res Tr 1998; 52: 175–184.
Bourguignon LY, Wong G, Earle C, Krueger K, Spevak CC . Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up-regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion. J Biol Chem 2010; 285: 36721–36735.
Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF . Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun 2005; 336: 1023–1027.
Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM . Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell 1997; 91: 949–960.
Mak P, Leung YK, Tang WY, Harwood C, Ho SM . Apigenin suppresses cancer cell growth through ERbeta. Neoplasia 2006; 8: 896–904.
Acknowledgements
NIH Grants CA80789 and 89209 supported this work. We thank Dr Xiofang Yang for her input into this project, and Drs Paul Mak and Hira Lal Goel for their valuable suggestions and technical assistance. We also thank Bryan Pursell for his technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Samanta, S., Sharma, V., Khan, A. et al. Regulation of IMP3 by EGFR signaling and repression by ERβ: implications for triple-negative breast cancer. Oncogene 31, 4689–4697 (2012). https://doi.org/10.1038/onc.2011.620
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.620
Keywords
This article is cited by
-
IGF2BP3 promotes the progression of colorectal cancer and mediates cetuximab resistance by stabilizing EGFR mRNA in an m6A-dependent manner
Cell Death & Disease (2023)
-
Targeting CXCL16 and STAT1 augments immune checkpoint blockade therapy in triple-negative breast cancer
Nature Communications (2023)
-
Lipidomic and Membrane Mechanical Signatures in Triple-Negative Breast Cancer: Scope for Membrane-Based Theranostics
Molecular and Cellular Biochemistry (2022)
-
The emerging role of RNA N6-methyladenosine methylation in breast cancer
Biomarker Research (2021)
-
The biological function of IGF2BPs and their role in tumorigenesis
Investigational New Drugs (2021)