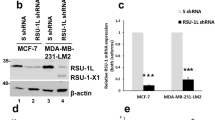
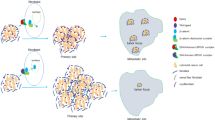
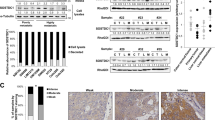
Abstract
The membrane-anchored matrix metalloproteinase-regulator RECK is often downregulated in cancers; in some cases, a significant correlation between the level of residual RECK in resected tumors and patient survival has been noted. Furthermore, restoration of RECK expression in certain cancer-derived cell lines results in reduced tumorigenicity. Here we report that acute RECK expression in colon carcinoma cells results in cell cycle-arrest accompanied by downregulation of a ubiquitin ligase component, S-phase kinase-associated protein 2 (SKP2), and upregulation of its substrate, p27KIP1. Our data indicate that RECK-induced growth suppression is at least partially dependent on p27, and that RECK and type I collagen share similar effects on the SKP2–p27 pathway. Importantly, in patients with lung, colorectal and bladder cancers, the RECK/SKP2 ratio is high in normal tissues and lower in the cancer tissues. These findings reveal a novel molecular pathway linking cell-cycle progression to RECK downregulation, extracellular matrix degradation and SKP2 upregulation, and suggest that treatment regimens that induce RECK expression could be promising cancer therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Bond M, Wu YJ, Sala-Newby GB, Newby AC . (2008). Rho GTPase, Rac1, regulates SKP2 levels, vascular smooth muscle cell proliferation, and intima formation in vitro and in vivo. Cardiovasc Res 80: 290–298.
Caswell PT, Vadrevu S, Norman JC . (2009). Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 10: 843–853.
Chandana EP, Maeda Y, Ueda A, Kiyonari H, Oshima N, Yamamoto M et al. (2010). Involvement of the Reck tumor suppressor protein in maternal and embryonic vascular remodeling in mice. BMC Dev Biol 10: 84.
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C et al. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367.
Dyrskjot L, Kruhoffer M, Thykjaer T, Marcussen N, Jensen JL, Moller K et al. (2004). Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res 64: 4040–4048.
Frescas D, Pagano M . (2008). Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 8: 438–449.
Freshney RI . (1987). Disaggregation of the tissue and primary culture. Culture of animal cells: A manual of basic technique 2nd edn. Wiley-Liss, Inc.: New York, pp 117–118.
Fu Y, Fang Z, Liang Y, Zhu X, Prins P, Li Z et al. (2007). Overexpression of integrin beta1 inhibits proliferation of hepatocellular carcinoma cell SMMC-7721 through preventing SKP2-dependent degradation of p27 via PI3K pathway. J Cell Biochem 102: 704–718.
Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ et al. (1996). Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem 271: 695–701.
Hatta M, Matsuzaki T, Morioka Y, Yoshida Y, Noda M . (2009). Density- and serum-dependent regulation of the Reck tumor suppressor in mouse embryo fibroblasts. Cell Signal 21: 1885–1893.
Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K . (1995). Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375: 500–503.
Kondo S, Shukunami C, Morioka Y, Matsumoto N, Takahashi R, Oh J et al. (2007). Dual effects of the membrane-anchored MMP regulator RECK on chondrogenic differentiation of ATDC5 cells. J Cell Sci 120: 849–857.
Landi MT, Dracheva T, Rotunno M, Figueroa JD, Liu H, Dasgupta A et al. (2008). Gene expression signature of cigarette smoking and its role in lung adenocarcinoma development and survival. PLoS One 3: e1651.
Leibovitz A, Stinson JC, McCombs 3rd WB, McCoy CE, Mazur KC, Mabry ND . (1976). Classification of human colorectal adenocarcinoma cell lines. Cancer Res 36: 4562–4569.
Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH et al. (2010). SKP2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 464: 374–379.
Loayza-Puch F, Yoshida Y, Matsuzaki T, Takahashi C, Kitayama H, Noda M . (2010). Hypoxia and RAS-signaling pathways converge on, and cooperatively downregulate, the RECK tumor-suppressor protein through microRNAs. Oncogene 29: 2638–2648.
Miki T, Takegami Y, Okawa K, Muraguchi T, Noda M, Takahashi C . (2007). The reversion-inducing cysteine-rich protein with Kazal motifs (RECK) interacts with membrane type 1 matrix metalloproteinase and CD13/aminopeptidase N and modulates their endocytic pathways. J Biol Chem 282: 12341–12352.
Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K et al. (1996). Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA 93: 1320–1324.
Morioka Y, Monypenny J, Matsuzaki T, Shi S, Alexander DB, Kitayama H et al. (2009). The membrane-anchored metalloproteinase regulator RECK stabilizes focal adhesions and anterior-posterior polarity in fibroblasts. Oncogene 28: 1454–1464.
Muraguchi T, Takegami Y, Ohtsuka T, Kitajima S, Chandana EP, Omura A et al. (2007). RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity. Nat Neurosci 10: 838–845.
Murai R, Yoshida Y, Muraguchi T, Nishimoto E, Morioka Y, Kitayama H et al. (2010). A novel screen using the Reck tumor suppressor gene promoter detects both conventional and metastasis-suppressing anticancer drugs. Oncotarget 1: 252–264.
Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K et al. (2000). Targeted disruption of SKP2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J 19: 2069–2081.
Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S et al. (2004). SKP2-mediated degradation of p27 regulates progression into mitosis. Dev Cell 6: 661–672.
Noda M, Kitayama H, Matsuzaki T, Sugimoto Y, Okayama H, Bassin RH et al. (1989). Detection of genes with a potential for suppressing the transformed phenotype associated with activated ras genes. Proc Natl Acad Sci USA 86: 162–166.
Noda M, Oh J, Takahashi R, Kondo S, Kitayama H, Takahashi C . (2003). RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev 22: 167–175.
Noda M, Selinger Z, Scolnick EM, Bassin RH . (1983). Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci USA 80: 5602–5606.
Noda M, Takahashi C . (2007). Recklessness as a hallmark of aggressive cancer. Cancer Sci 98: 1659–1665.
Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM et al. (2001). The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107: 789–800.
Omura A, Matsuzaki T, Mio K, Ogura T, Yamamoto M, Fujita A et al. (2009). RECK forms cowbell-shaped dimers and inhibits matrix metalloproteinase-catalyzed cleavage of fibronectin. J Biol Chem 284: 3461–3469.
Prifti E, Zucker JD, Clement K, Henegar C . (2008). FunNet: an integrative tool for exploring transcriptional interactions. Bioinformatics 24: 2636–2638.
Rasheed S, Nelson-Rees WA, Toth EM, Arnstein P, Gardner MB . (1974). Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer 33: 1027–1033.
Sabates-Bellver J, Van der Flier LG, de Palo M, Cattaneo E, Maake C, Rehrauer H et al. (2007). Transcriptome profile of human colorectal adenomas. Mol Cancer Res 5: 1263–1275.
Sasahara RM, Takahashi C, Noda M . (1999). Involvement of the Sp1 site in ras-mediated downregulation of the RECK metastasis suppressor gene. Biochem Biophys Res Commun 264: 668–675.
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW . (1997). Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593–602.
Sternlicht MD, Werb Z . (2001). How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 17: 463–516.
Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K et al. (1998). Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci USA 95: 13221–13226.
Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M et al. (1991). The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781.
Yoshida Y, Hamada H . (1997). Adenovirus-mediated inducible gene expression through tetracycline-controllable transactivator with nuclear localization signal. Biochem Biophys Res Commun 230: 426–430.
Zhang H, Ozaki I, Mizuta T, Yoshimura T, Matsuhashi S, Hisatomi A et al. (2003). Mechanism of beta 1-integrin-mediated hepatoma cell growth involves p27 and S-phase kinase-associated protein 2. Hepatology 38: 305–313.
Zhu L . (2010). SKP2 knockout reduces cell proliferation and mouse body size: and prevents cancer? Cell Res 20: 605–607.
Acknowledgements
We thank Chiaki Takahashi for the RECK cDNA, Mako Yamamoto for R1B2-6 cells, Hitoshi Kitayama and Tomoko Matsuzaki for discussions, David Alexander for critical reading of the manuscript, Hai-Ou Gu and Aiko Nishimoto for technical assistance and Aki Miyazaki for secretarial assistance. This work was supported by JSPS Grant-in-Aid for Creative Scientific Research and MEXT Grant-in-Aid on Priority Areas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yoshida, Y., Ninomiya, K., Hamada, H. et al. Involvement of the SKP2–p27KIP1 pathway in suppression of cancer cell proliferation by RECK. Oncogene 31, 4128–4138 (2012). https://doi.org/10.1038/onc.2011.570
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.570
Keywords
This article is cited by
-
Suppression of tumor metastasis by a RECK-activating small molecule
Scientific Reports (2022)
-
EAG1 enhances hepatocellular carcinoma proliferation by modulating SKP2 and metastasis through pseudopod formation
Oncogene (2021)
-
Analysis of the inhibiting activity of reversion-inducing cysteine-rich protein with Kazal motifs (RECK) on matrix metalloproteinases
Scientific Reports (2020)
-
RECK (reversion-inducing cysteine-rich protein with Kazal motifs) regulates migration, differentiation and Wnt/β-catenin signaling in human mesenchymal stem cells
Cellular and Molecular Life Sciences (2016)
-
Critical roles for murine Reck in the regulation of vascular patterning and stabilization
Scientific Reports (2015)