Abstract
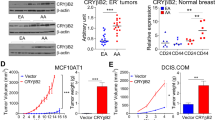
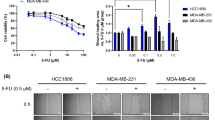
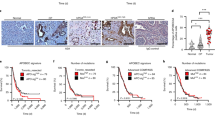
Alpha B-crystallin (CRYAB) maps within the nasopharyngeal carcinoma (NPC) tumor-suppressive critical region 11q22-23 and its downregulation is significantly associated with the progression of NPC. However, little is known about the functional impact of CRYAB on NPC progression. In this study we evaluated the NPC tumor-suppressive and progression-associated functions of CRYAB. Activation of CRYAB suppressed NPC tumor formation in nude mice. Overexpression of CRYAB affected NPC progression-associated phenotypes such as loss of cell adhesion, invasion, interaction with the tumor microenvironment, invasive protrusion formation in three dimensional Matrigel culture, as well as expression of epithelial–mesenchymal transition-associated markers. CRYAB mediates this ability to suppress cancer progression by inhibition of E-cadherin cytoplasmic internalization and maintenance of β-catenin in the membrane that subsequently reduces the levels of expression of critical downstream targets such as cyclin-D1 and c-myc. Both ectopically expressed and recombinant CRYAB proteins were associated with endogenous E-cadherin and β-catenin, and, thus, the cadherin/catenin adherens junction. The CRYAB α-crystallin core domain is responsible for the interaction of CRYAB with both E-cadherin and β-catenin. Taken together, these results indicate that CRYAB functions to suppress NPC progression by associating with the cadherin/catenin adherens junction and modulating the β-catenin function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cheng Y, Stanbridge EJ, Kong H, Bengtsson U, Lerman MI, Lung ML . (2000). A functional investigation of tumor suppressor gene activities in a nasopharyngeal carcinoma cell line HONE1 using a monochromosome transfer approach. Genes Chromosomes Cancer 28: 82–91.
Chetty R, Serra S . (2008). Nuclear E-cadherin immunoexpression: from biology to potential applications in diagnostic pathology. Adv Anat Pathol 15: 234–240.
Cheung AK, Lung HL, Ko JM, Cheng Y, Stanbridge EJ, Zabarovsky ER et al. (2009). Chromosome 14 transfer and functional studies identify a candidate tumor suppressor gene, mirror image polydactyly 1, in nasopharyngeal carcinoma. Proc Natl Acad Sci USA 106: 14478–14483.
Cheung ST, Huang DP, Hui AB, Lo KW, Ko CW, Tsang YS et al. (1999). Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein–Barr virus. Int J Cancer 83: 121–126.
Chou J, Lin YC, Kim J, You L, Xu Z, He B et al. (2008). Nasopharyngeal carcinoma—review of the molecular mechanisms of tumorigenesis. Head Neck 30: 946–963.
Ghosh JG, Houck SA, Clark JI . (2007a). Interactive sequences in the stress protein and molecular chaperone human alphaB crystallin recognize and modulate the assembly of filaments. Int J Biochem Cell Biol 39: 1804–1815.
Ghosh JG, Shenoy Jr AK, Clark JI . (2007b). Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry 46: 6308–6317.
Green KJ, Getsios S, Troyanovsky S, Godsel LM . (2010). Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol 2: a000125.
Grunert S, Jechlinger M, Beug H . (2003). Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol 4: 657–665.
Heuberger J, Birchmeier W . (2010). Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2: a002915.
Hui AB, Lo KW, Leung SF, Choi PH, Fong Y, Lee JC et al. (1996). Loss of heterozygosity on the long arm of chromosome 11 in nasopharyngeal carcinoma. Cancer Res 56: 3225–3229.
Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, Liang Y et al. (2010). Epstein–Barr virus-encoded LMP2A induces an epithelial–mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog 6: e1000940.
Lee JM, Dedhar S, Kalluri R, Thompson EW . (2006). The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172: 973–981.
Leung AC, Wong VC, Yang LC, Chan PL, Daigo Y, Nakamura Y et al. (2008). Frequent decreased expression of candidate tumor suppressor gene, DEC1, and its anchorage-independent growth properties and impact on global gene expression in esophageal carcinoma. Int J Cancer 122: 587–594.
Li HM, Man C, Jin Y, Deng W, Yip YL, Feng HC et al. (2006). Molecular and cytogenetic changes involved in the immortalization of nasopharyngeal epithelial cells by telomerase. Int J Cancer 119: 1567–1576.
Li J, Wang F, Protopopov A, Malyukova A, Kashuba V, Minna JD et al. (2004). Inactivation of RASSF1C during in vivo tumor growth identifies it as a tumor suppressor gene. Oncogene 23: 5941–5949.
Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ et al. (2006). Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF (FBX4–alphaB crystallin) complex. Mol Cell 24: 355–366.
Lin JC, Liao SK, Lee EH, Hung MS, Sayion Y, Chen HC et al. (2009). Molecular events associated with epithelial to mesenchymal transition of nasopharyngeal carcinoma cells in the absence of Epstein–Barr virus genome. J Biomed Sci 16: 105.
Lo AK, Liu Y, Wang XH, Huang DP, Yuen PW, Wong YC et al. (2003). Alterations of biologic properties and gene expression in nasopharyngeal epithelial cells by the Epstein–Barr virus-encoded latent membrane protein 1. Lab Invest 83: 697–709.
Lo KW, Teo PM, Hui AB, To KF, Tsang YS, Chan SY et al. (2000). High resolution allelotype of microdissected primary nasopharyngeal carcinoma. Cancer Res 60: 3348–3353.
Lu Z, Ghosh S, Wang Z, Hunter T . (2003). Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 4: 499–515.
Lung HL, Bangarusamy DK, Xie D, Cheung AK, Cheng Y, Kumaran MK et al. (2005). THY1 is a candidate tumour suppressor gene with decreased expression in metastatic nasopharyngeal carcinoma. Oncogene 24: 6525–6532.
Lung HL, Cheung AK, Cheng Y, Kwong FM, Lo PH, Law EW et al. (2010). Functional characterization of THY1 as a tumor suppressor gene with antiinvasive activity in nasopharyngeal carcinoma. Int J Cancer 127: 304–312.
Lung HL, Cheung AK, Xie D, Cheng Y, Kwong FM, Murakami Y et al. (2006). TSLC1 is a tumor suppressor gene associated with metastasis in nasopharyngeal carcinoma. Cancer Res 66: 9385–9392.
Lung HL, Lo CC, Wong CC, Cheung AK, Cheong KF, Wong N et al. (2008). Identification of tumor suppressive activity by irradiation microcell-mediated chromosome transfer and involvement of alpha B-crystallin in nasopharyngeal carcinoma. Int J Cancer 122: 1288–1296.
Maddala R, Rao VP . (2005). alpha-Crystallin localizes to the leading edges of migrating lens epithelial cells. Exp Cell Res 306: 203–215.
Nelson WJ, Nusse R . (2004). Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303: 1483–1487.
Ohto-Fujita E, Fujita Y, Atomi Y . (2007). Analysis of the alphaB-crystallin domain responsible for inhibiting tubulin aggregation. Cell Stress Chaperones 12: 163–171.
Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C . (2005). Lysosomal targeting of E-cadherin: a unique mechanism for the downregulation of cell–cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol 25: 389–402.
Protopopov AI, Li J, Winberg G, Gizatullin RZ, Kashuba VI, Klein G et al. (2002). Human cell lines engineered for tetracycline-regulated expression of tumor suppressor candidate genes from a frequently affected chromosomal region, 3p21. J Gene Med 4: 397–406.
Reynolds AB, Roczniak-Ferguson A . (2004). Emerging roles for p120–catenin in cell adhesion and cancer. Oncogene 23: 7947–7956.
Simon S, Fontaine JM, Martin JL, Sun X, Hoppe AD, Welsh MJ et al. (2007). Myopathy-associated alphaB-crystallin mutants: abnormal phosphorylation, intracellular location, and interactions with other small heat shock proteins. J Biol Chem 282: 34276–34287.
Sizhong Z, Xiukung G, Yi Z . (1983). Cytogenetic studies on an epithelial cell line derived from poorly differentiated nasopharyngeal carcinoma. Int J Cancer 31: 587–590.
Solares CA, Boyle GM, Brown I, Parsons PG, Panizza B . (2010). Reduced alphaB-crystallin staining in perineural invasion of head and neck cutaneous squamous cell carcinoma. Otolaryngol Head Neck Surg 142: S15–S19.
Stronach EA, Sellar GC, Blenkiron C, Rabiasz GJ, Taylor KJ, Miller EP et al. (2003). Identification of clinically relevant genes on chromosome 11 in a functional model of ovarian cancer tumor suppression. Cancer Res 63: 8648–8655.
Takashi M, Katsuno S, Sakata T, Ohshima S, Kato K . (1998). Different concentrations of two small stress proteins, alphaB crystallin and HSP27 in human urological tumor tissues. Urol Res 26: 395–399.
Thedieck C, Kalbacher H, Kratzer U, Lammers R, Stevanovic S, Klein G . (2008). alpha B-Crystallin is a cytoplasmic interaction partner of the kidney-specific cadherin-16. J Mol Biol 378: 145–153.
Thiery JP, Acloque H, Huang RY, Nieto MA . (2009). Epithelial–mesenchymal transitions in development and disease. Cell 139: 871–890.
Thomson S, Petti F, Sujka-Kwok I, Mercado P, Bean J, Monaghan M et al. (2011). A systems view of epithelial–mesenchymal transition signaling states. Clin Exp Metastasis 28: 137–155.
Wong VC, Chen H, Ko JM, Chan KW, Chan YP, Law S et al. (2012). Tumor suppressor Dual-specificity Phosphatase 6 (DUSP6) impairs cell invasion and epithelial–mesenchymal transition (EMT)-associated phenotype. Int J Cancer 130: 83–95.
Wong VC, Ko JM, Qi RZ, Li PJ, Wang LD, Li JL et al. (2011). Abrogated expression of DEC1 during oesophageal squamous cell carcinoma progression is age- and family history-related and significantly associated with lymph node metastasis. Br J Cancer 104: 841–849.
Zhang J, Yu L, Wu X, Zou L, Sou KK, Wei Z et al. (2010). The interacting domains of hCdt1 and hMcm6 involved in the chromatin loading of the MCM complex in human cells. Cell Cycle 9: 4848–4857.
Zheng Z, Pan J, Chu B, Wong YC, Cheung AL, Tsao SW . (1999). Downregulation and abnormal expression of E-cadherin and beta-catenin in nasopharyngeal carcinoma: close association with advanced disease stage and lymph node metastasis. Hum Pathol 30: 458–466.
Acknowledgements
We thank Patrick Vicart for the pcDNA3-CRYAB construct. We acknowledge the funding support from the Research Grants Council grants, and the University Grants Council of Hong Kong Special Administrative Region, People's Republic of China, for AoE/M-06/08 to MLL; The University of Hong Kong Seed Funding Programme for Basic Research to HLL; and the Swedish Cancer Society, the Swedish Research Council, the Swedish Institute and Karolinska Institute to ERZ. We acknowledge the Area of Excellence Hong Kong NPC Research Tissue Bank for the NPC specimens and tissue blocks.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Huang, Z., Cheng, Y., Chiu, P. et al. Tumor suppressor Alpha B-crystallin (CRYAB) associates with the cadherin/catenin adherens junction and impairs NPC progression-associated properties. Oncogene 31, 3709–3720 (2012). https://doi.org/10.1038/onc.2011.529
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.529
Keywords
This article is cited by
-
Splicing factor derived circular RNA circCAMSAP1 accelerates nasopharyngeal carcinoma tumorigenesis via a SERPINH1/c-Myc positive feedback loop
Molecular Cancer (2022)
-
Transcriptomics indicate nuclear division and cell adhesion not recapitulated in MCF7 and MCF10A compared to luminal A breast tumours
Scientific Reports (2022)
-
Identification of CELSR2 as a novel prognostic biomarker for hepatocellular carcinoma
BMC Cancer (2020)
-
Prognostic value of biomarkers EpCAM and αB-crystallin associated with lymphatic metastasis in breast cancer by iTRAQ analysis
BMC Cancer (2019)
-
Integrative analysis of transcriptomics and clinical data uncovers the tumor-suppressive activity of MITF in prostate cancer
Cell Death & Disease (2018)