Abstract
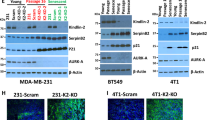
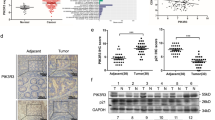
Senescence is an irreversible growth arrest phenotype adopted by cells that has a key role in protecting organisms from cancer. There is now considerable interest in therapeutic strategies that reactivate this process to control the growth of cancer cells. Protein kinase-Cι (PKCι) is a member of the atypical PKC family and an important downstream mediator in the phosphoinositide-3-kinase (PI-3-kinase) pathway. PKCι expression was found to be upregulated in a subset of breast cancers and breast cancer cell lines. Activation of the PI-3-kinase pathway by introduction of mutant, oncogenic PIK3CA into breast mammary epithelial cells increased both the expression and activation of PKCι. In breast cancer cells lines overexpressing PKCι, depletion of PKCι increased the number of senescent cells, as assessed by senescence-associated β-galactosidase, morphology and bromodeoxyuridine incorporation. This phenomenon was not restricted to breast cancer cells, as it was also seen in glioblastoma cells in which PKCι is activated by loss of PTEN. Senescence occurred in the absence of a detectable DNA-damage response, was dependent on p21 and was enhanced by the aurora kinase inhibitor VX-680, suggesting that senescence is triggered by defects in mitosis. Depletion of PKCι had no effect on senescence in normal mammary epithelial cell lines. We conclude that PKCι is overexpressed in a subset of cancers where it functions to suppress premature senescence. This function appears to be restricted to cancer cells and inhibition of PKCι may therefore be an effective way to selectively activate premature senescence in cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A et al. (2004). BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet 36: 744–749.
Baldwin RM, Barrett GM, Parolin DA, Gillies JK, Paget JA, Lavictoire SJ et al. (2010). Coordination of glioblastoma cell motility by PKCiota. Mol Cancer 9: 233.
Baldwin RM, Garratt-Lalonde M, Parolin DA, Krzyzanowski PM, Andrade MA, Lorimer IA . (2006). Protection of glioblastoma cells from cisplatin cytotoxicity via protein kinase Ciota-mediated attenuation of p38 MAP kinase signaling. Oncogene 25: 2909–2919.
Baldwin RM, Parolin DA, Lorimer IA . (2008). Regulation of glioblastoma cell invasion by PKC iota and RhoB. Oncogene 27: 3587–3595.
Balendran A, Biondi RM, Cheung PC, Casamayor A, Deak M, Alessi DR . (2000). A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Czeta (PKCzeta) and PKC-related kinase 2 by PDK1. J Biol Chem 275: 20806–20813.
Barr AR, Gergely F . (2007). Aurora-A: the maker and breaker of spindle poles. J Cell Sci 120: 2987–2996.
Bornancin F, Parker PJ . (1996). Phosphorylation of threonine 638 critically controls the dephosphorylation and inactivation of protein kinase Calpha. Curr Biol 6: 1114–1123.
Carracedo A, Alimonti A, Pandolfi PP . (2011). PTEN level in tumor suppression: how much is too little? Cancer Res 71: 629–633.
Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M et al. (2005). Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436: 725–730.
Collado M, Medema RH, Garcia-Cao I, Dubuisson ML, Barradas M, Glassford J et al. (2000). Inhibition of the phosphoinositide 3-kinase pathway induces a senescence-like arrest mediated by p27Kip1. J Biol Chem 275: 21960–21968.
Crowder RJ, Phommaly C, Tao Y, Hoog J, Luo J, Perou CM et al. (2009). PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res 69: 3955–3962.
di Fagagna FA, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, von Zglinicki T et al. (2003). A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198.
Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky Jr WE et al. (2009). Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 41: 544–552.
Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O . (2009). Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4: 1798–1806.
Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR et al. (2006). Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol 8: 1053–1063.
Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C et al. (2006). Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444: 638–642.
Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP et al. (2005). Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci USA 102: 12519–12524.
Fields AP, Regala RP . (2007). Protein kinase C iota: human oncogene, prognostic marker and therapeutic target. Pharmacol Res 55: 487–497.
Freeley M, Kelleher D, Long A . (2011). Regulation of protein kinase C function by phosphorylation on conserved and non-conserved sites. Cell Signal 23: 753–762.
Gao T, Furnari F, Newton AC . (2005). PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell 18: 13–24.
Gewirtz DA, Holt SE, Elmore LW . (2008). Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol 76: 947–957.
Gorgun G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M et al. (2010). A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood 115: 5202–5213.
Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T et al. (2004). VX-680 a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med 10: 262–267.
Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE et al. (1995). Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res 55: 4525–4530.
Huck JJ, Zhang M, McDonald A, Bowman D, Hoar KM, Stringer B et al. (2010). MLN8054, an inhibitor of Aurora A kinase, induces senescence in human tumor cells both in vitro and in vivo. Mol Cancer Res 8: 373–384.
Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV et al. (2005). Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res 65: 10992–11000.
Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, Van Meir EG . (1999). Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol 9: 469–479.
Kanzaki M, Mora S, Hwang JB, Saltiel AR, Pessin JE . (2004). Atypical protein kinase C (PKCzeta/lambda) is a convergent downstream target of the insulin-stimulated phosphatidylinositol 3-kinase and TC10 signaling pathways. J Cell Biol 164: 279–290.
Karakas B, Bachman KE, Park BH . (2006). Mutation of the PIK3CA oncogene in human cancers. Br J Cancer 94: 455–459.
Kojima Y, Akimoto K, Nagashima Y, Ishiguro H, Shirai S, Chishima T et al. (2008). The overexpression and altered localization of the atypical protein kinase C lambda/iota in breast cancer correlates with the pathologic type of these tumors. Hum Pathol 39: 824–831.
Lawless C, Wang C, Jurk D, Merz A, Zglinicki TV, Passos JF . (2010). Quantitative assessment of markers for cell senescence. Exp Gerontol 45: 772–778.
Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH et al. (2010). Skp2 targeting suppresses tumorigenesis by Arf–p53-independent cellular senescence. Nature 464: 374–379.
Lorimer IA, Lavictoire SJ . (2000). Targeting retrovirus to cancer cells expressing a mutant EGF receptor by insertion of a single chain antibody variable domain in the envelope glycoprotein receptor binding lobe. J Immunol Methods 237: 147–157.
Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM et al. (2005). BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436: 720–724.
Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC, Muthuswamy SK . (2008). The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res 68: 8201–8209.
O'Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M et al. (1997). Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res 57: 4285–4300.
Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ et al. (2010). Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6: 347.
Pearce LR, Komander D, Alessi DR . (2010). The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22.
Peifer C, Alessi DR . (2008). Small-molecule inhibitors of PDK1. Chem Med Chem 3: 1810–1838.
Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM et al. (2003). High frequency of BRAF mutations in nevi. Nat Genet 33: 19–20.
Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM, Fields AP . (2005). Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res 65: 8905–8911.
Schmitt CA . (2007). Cellular senescence and cancer treatment. Biochim Biophys Acta 1775: 5–20.
Scott MT, Ingram A, Ball KL . (2002). PDK1-dependent activation of atypical PKC leads to degradation of the p21 tumour modifier protein. EMBO J 21: 6771–6780.
Scotti ML, Bamlet WR, Smyrk TC, Fields AP, Murray NR . (2010). Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res 70: 2064–2074.
Vanhaesebroeck B, Alessi DR . (2000). The PI3K–PDK1 connection: more than just a road to PKB. Biochem J 346: 561–576.
Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ et al. (2009). AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 16: 21–32.
Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L et al. (2007). Restoration of p53 function leads to tumour regression in vivo. Nature 445: 661–665.
Wu CH, van Riggelen J, Yetil A, Fan AC, Bachireddy P, Felsher DW . (2007). Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci USA 104: 13028–13033.
Xu L, Deng X . (2006). Protein kinase Ciota promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of micro- and m-calpains. J Biol Chem 281: 4457–4466.
Yuan TL, Wulf G, Burga L, Cantley LC . (2011). Cell-to-cell variability in PI3K protein level regulates PI3K–AKT pathway activity in cell populations. Curr Biol 21: 173–183.
Zhang L, Huang J, Yang N, Liang S, Barchetti A, Giannakakis A et al. (2006). Integrative genomic analysis of protein kinase C (PKC) family identifies PKCiota as a biomarker and potential oncogene in ovarian carcinoma. Cancer Res 66: 4627–4635.
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research and the Ottawa Regional Cancer Foundation (to IAJL). JP was supported by a Canadian Institutes of Health Research Banting and Best MSc Scholarship. IR is supported by an Ontario Graduate Scholarship in Science and Technology. We thank Sharon Rowan and Mara Coiciu for their work on the initial characterization of PKCι activation in breast cancer cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Paget, J., Restall, I., Daneshmand, M. et al. Repression of cancer cell senescence by PKCι. Oncogene 31, 3584–3596 (2012). https://doi.org/10.1038/onc.2011.524
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.524
Keywords
This article is cited by
-
The therapeutic potential of Aurora kinases targeting in glioblastoma: from preclinical research to translational oncology
Journal of Molecular Medicine (2020)
-
Induction of senescence in primary glioblastoma cells by serum and TGFβ
Scientific Reports (2017)
-
Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses?
Cellular and Molecular Life Sciences (2017)
-
PKCiota promotes ovarian tumor progression through deregulation of cyclin E
Oncogene (2016)
-
Involvement of Tight Junction Plaque Proteins in Cancer
Current Pathobiology Reports (2016)