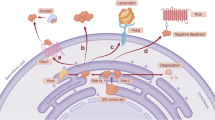
Abstract
The von Hippel–Lindau tumor suppressor gene (VHL) has attracted intensive interest not only because its mutations predispose carriers to devastating tumors, but also because it is involved in oxygen sensing under physiological conditions. VHL loss-of-function mutations result in organ-specific tumors, such as hemangioblastoma of the central nervous system and renal cell carcinoma, both untreatable with conventional chemotherapies. The VHL protein is best known as an E3 ubiquitin ligase that targets hypoxia-inducible factor-α (HIF-α), but many diverse, non-canonical cellular functions have also been assigned to VHL, mainly based on studies in cell culture systems. As such, although the HIF-dependent role of VHL is critical, the full spectrum of pathophysiological functions of VHL is still unresolved. Such understanding requires careful cross-referencing with physiologically relevant experimental models. Studies in model systems, such as Caenorhabditis elegans, Drosophila, zebrafish and mouse have provided critical in vivo confirmation of the VHL–HIF pathway, and verification of potentially important cellular functions including microtubule stabilization and epithelial morphogenesis. More recently, animal models have also suggested systemic roles of VHL in hematopoiesis, metabolic homeostasis and inflammation. In this review, the studies performed in model organisms will be summarized and placed in context with existing clinical and in vitro data.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Adryan B, Decker HJ, Papas TS, Hsu T . (2000). Tracheal development and the von Hippel-Lindau tumor suppressor homolog in Drosophila. Oncogene 19: 2803–2811.
Akis N, Madaio MP . (2004). Isolation, culture, and characterization of endothelial cells from mouse glomeruli. Kidney Int 65: 2223–2227.
Alexander A, Walker CL . (2011). The role of LKB1 and AMPK in cellular responses to stress and damage. FEBS Lett 585: 952–957.
Arquier N, Vigne P, Duplan E, Hsu T, Therond PP, Frelin C et al. (2006). Analysis of the hypoxia-sensing pathway in Drosophila melanogaster. Biochem J 393: 471–480.
Aso T, Yamazaki K, Aigaki T, Kitajima S . (2000). Drosophila von Hippel-Lindau tumor suppressor complex possesses E3 ubiquitin ligase activity. Biochem Biophys Res Commun 276: 355–361.
Banks RE, Tirukonda P, Taylor C, Hornigold N, Astuti D, Cohen D et al. (2006). Genetic and epigenetic analysis of von Hippel-Lindau (VHL) gene alterations and relationship with clinical variables in sporadic renal cancer. Cancer Res 66: 2000–2011.
Bartolini F, Gundersen GG . (2006). Generation of noncentrosomal microtubule arrays. J Cell Sci 119: 4155–4163.
Behr M, Wingen C, Wolf C, Schuh R, Hoch M . (2007). Wurst is essential for airway clearance and respiratory-tube size control. Nat Cell Biol 9: 847–853.
Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J . (2003). HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082–4090.
Betschinger J, Eisenhaber F, Knoblich JA . (2005). Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr Biol 15: 276–282.
Biju MP, Neumann AK, Bensinger SJ, Johnson RS, Turka LA, Haase VH . (2004). Vhlh gene deletion induces Hif-1-mediated cell death in thymocytes. Mol Cell Biol 24: 9038–9047.
Bilder D, Schober M, Perrimon N . (2003). Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol 5: 53–58.
Bishop T, Lau KW, Epstein AC, Kim SK, Jiang M, O'Rourke D et al. (2004). Genetic analysis of pathways regulated by the von Hippel-Lindau tumor suppressor in Caenorhabditis elegans. PLoS Biol 2: e289.
Brauch H, Kishida T, Glavac D, Chen F, Pausch F, Hofler H et al. (1995). Von Hippel-Lindau (VHL) disease with pheochromocytoma in the Black Forest region of Germany: evidence for a founder effect. Hum Genet 95: 551–556.
Brukamp K, Jim B, Moeller MJ, Haase VH . (2007). Hypoxia and podocyte-specific Vhlh deletion confer risk of glomerular disease. Am J Physiol Renal Physiol 293: F1397–F1407.
Bushuev VI, Miasnikova GY, Sergueeva AI, Polyakova LA, Okhotin D, Gaskin PR et al. (2006). Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica 91: 744–749.
Centanin L, Dekanty A, Romero N, Irisarri M, Gorr TA, Wappner P . (2008). Cell autonomy of HIF effects in Drosophila: tracheal cells sense hypoxia and induce terminal branch sprouting. Dev Cell 14: 547–558.
Champion KJ, Guinea M, Dammai V, Hsu T . (2008). Endothelial function of von Hippel-Lindau tumor suppressor gene: control of fibroblast growth factor receptor signaling. Cancer Res 68: 4649–4657.
Cho D, Signoretti S, Regan M, Mier JW, Atkins MB . (2007). The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res 13: 758s–7763s.
Clark PE . (2009). The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney Int 76: 939–945.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A . (2009). Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30: 1073–1081.
Dammai V, Adryan B, Lavenburg KR, Hsu T . (2003). Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev 17: 2812–2824.
Danilin S, Sourbier C, Thomas L, Rothhut S, Lindner V, Helwig JJ et al. (2009). von Hippel-Lindau tumor suppressor gene-dependent mRNA stabilization of the survival factor parathyroid hormone-related protein in human renal cell carcinoma by the RNA-binding protein HuR. Carcinogenesis 30: 387–396.
Datta K, Mondal S, Sinha S, Li J, Wang E, Knebelmann B et al. (2005). Role of elongin-binding domain of von Hippel Lindau gene product on HuR-mediated VPF/VEGF mRNA stability in renal cell carcinoma. Oncogene 24: 7850–7858.
Doronkin S, Djagaeva I, Nagle ME, Reiter LT, Seagroves TN . (2010). Dose-dependent modulation of HIF-1alpha/sima controls the rate of cell migration and invasion in Drosophila ovary border cells. Oncogene 29: 1123–1134.
Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P . (2001). Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107: 17–26.
Duchi S, Fagnocchi L, Cavaliere V, Hsouna A, Gargiulo G, Hsu T . (2010). Drosophila VHL tumor-suppressor gene regulates epithelial morphogenesis by promoting microtubule and aPKC stability. Development 137: 1493–1503.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR et al. (2001). C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54.
Esteban MA, Harten SK, Tran MG, Maxwell PH . (2006). Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J Am Soc Nephrol 17: 1801–1806.
Feijoo-Cuaresma M, Mendez F, Maqueda A, Esteban MA, Naranjo-Suarez S, Castellanos MC et al. (2008). Inadequate activation of the GTPase RhoA contributes to the lack of fibronectin matrix assembly in von Hippel-Lindau protein-defective renal cancer cells. J Biol Chem 283: 24982–24990.
Frew IJ, Krek W . (2007). Multitasking by pVHL in tumour suppression. Curr Opin Cell Biol 19: 685–690.
Frew IJ, Krek W . (2008). pVHL: a multipurpose adaptor protein. Sci Signal 1: pe30.
Frew IJ, Minola A, Georgiev S, Hitz M, Moch H, Richard S et al. (2008a). Combined VHLH and PTEN mutation causes genital tract cystadenoma and squamous metaplasia. Mol Cell Biol 28: 4536–4548.
Frew IJ, Thoma CR, Georgiev S, Minola A, Hitz M, Montani M et al. (2008b). pVHL and PTEN tumour suppressor proteins cooperatively suppress kidney cyst formation. EMBO J 27: 1747–1757.
Gao J, Naglich JG, Laidlaw J, Whaley JM, Seizinger BR, Kley N . (1995). Cloning and characterization of a mouse gene with homology to the human von Hippel-Lindau disease tumor suppressor gene: implications for the potential organization of the human von Hippel-Lindau disease gene. Cancer Res 55: 743–747.
Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA . (2003). Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol 19: 623–647.
Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A et al. (1997). Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA 94: 9102–9107.
Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL et al. (2004). Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood 103: 3924–3932.
Grivennikov SI, Greten FR, Karin M . (2010). Immunity, inflammation, and cancer. Cell 140: 883–899.
Guo Y, Schoell MC, Freeman RS . (2009). The von Hippel-Lindau protein sensitizes renal carcinoma cells to apoptotic stimuli through stabilization of BIM(EL). Oncogene 28: 1864–1874.
Haase VH, Glickman JN, Socolovsky M, Jaenisch R . (2001). Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci USA 98: 1583–1588.
Harten SK, Shukla D, Barod R, Hergovich A, Balda MS, Matter K et al. (2009). Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin. Mol Biol Cell 20: 1089–1101.
Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W . (2003). Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol 5: 64–70.
Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S et al. (1994). Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci USA 91: 9700–9704.
Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H et al. (2010). The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest 120: 827–839.
Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B et al. (2007). Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820.
Hong SB, Furihata M, Baba M, Zbar B, Schmidt LS . (2006). Vascular defects and liver damage by the acute inactivation of the VHL gene during mouse embryogenesis. Lab Invest 86: 664–675.
Hsouna A, Nallamothu G, Kose N, Guinea M, Dammai V, Hsu T . (2010). Drosophila von Hippel-Lindau tumor suppressor gene function in epithelial tubule morphogenesis. Mol Cell Biol 30: 3779–3794.
Hsu T, Adereth Y, Kose N, Dammai V . (2006). Endocytic function of von Hippel-Lindau tumor suppressor protein regulates surface localization of fibroblast growth factor receptor 1 and cell motility. J Biol Chem 281: 12069–12080.
Hussain SP, Hofseth LJ, Harris CC . (2003). Radical causes of cancer. Nat Rev Cancer 3: 276–285.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M et al. (2001). HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ et al. (2001). Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472.
Jarecki J, Johnson E, Krasnow MA . (1999). Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell 99: 211–220.
Kaelin WG . (2005). The von Hippel-Lindau tumor suppressor protein: roles in cancer and oxygen sensing. Cold Spring Harb Symp Quant Biol 70: 159–166.
Kaelin Jr WG . (2009). Treatment of kidney cancer: insights provided by the VHL tumor-suppressor protein. Cancer 115: 2262–2272.
Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, Iliopoulos O et al. (1999). Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284: 657–661.
Kapitsinou PP, Haase VH . (2008). The VHL tumor suppressor and HIF: insights from genetic studies in mice. Cell Death Differ 15: 650–659.
Kessler PM, Vasavada SP, Rackley RR, Stackhouse T, Duh FM, Latif F et al. (1995). Expression of the Von Hippel-Lindau tumor suppressor gene, VHL, in human fetal kidney and during mouse embryogenesis. Mol Med 1: 457–466.
Kim WY, Kaelin WG . (2004). Role of VHL gene mutation in human cancer. J Clin Oncol 22: 4991–5004.
Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K et al. (2008). Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 295: F1023–F1029.
Kraus S, Arber N . (2009). Inflammation and colorectal cancer. Curr Opin Pharmacol 9: 405–410.
Kurban G, Duplan E, Ramlal N, Hudon V, Sado Y, Ninomiya Y et al. (2008). Collagen matrix assembly is driven by the interaction of von Hippel-Lindau tumor suppressor protein with hydroxylated collagen IV alpha 2. Oncogene 27: 1004–1012.
Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML et al. (1993). Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260: 1317–1320.
Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W . (1999). The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev 13: 1822–1833.
Lonergan KM, Iliopoulos O, Ohh M, Kamura T, Conaway RC, Conaway JW et al. (1998). Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol Cell Biol 18: 732–741.
Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM et al. (2003). von Hippel-Lindau disease. Lancet 361: 2059–2067.
Ma W, Tessarollo L, Hong SB, Baba M, Southon E, Back TC et al. (2003). Hepatic vascular tumors, angiectasis in multiple organs, and impaired spermatogenesis in mice with conditional inactivation of the VHL gene. Cancer Res 63: 5320–5328.
Ma XM, Blenis J . (2009). Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318.
Maher ER, Neumann HP, Richard S . (2011). von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet 19: 617–623.
Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM et al. (2002). HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1: 459–468.
McDonald JA, Pinheiro EM, Montell DJ . (2003). PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development 130: 3469–3478.
Mikhaylova O, Ignacak ML, Barankiewicz TJ, Harbaugh SV, Yi Y, Maxwell PH et al. (2008). The von Hippel-Lindau tumor suppressor protein and Egl-9-Type proline hydroxylases regulate the large subunit of RNA polymerase II in response to oxidative stress. Mol Cell Biol 28: 2701–2717.
Montell DJ . (2003). Border-cell migration: the race is on. Nat Rev Mol Cell Biol 4: 13–24.
Mortimer NT, Moberg KH . (2009). Regulation of Drosophila embryonic tracheogenesis by dVHL and hypoxia. Dev Biol 329: 294–305.
Muller RU, Fabretti F, Zank S, Burst V, Benzing T, Schermer B . (2009). The von Hippel Lindau tumor suppressor limits longevity. J Am Soc Nephrol 20: 2513–2517.
Nallamothu G, Dammai V, Hsu T . (2009). Developmental function of Nm23/awd: a mediator of endocytosis. Mol Cell Biochem 329: 35–44.
Nallamothu G, Woolworth JA, Dammai V, Hsu T . (2008). Awd, the homolog of metastasis suppressor gene Nm23, regulates Drosophila epithelial cell invasion. Mol Cell Biol 28: 1964–1973.
Ohh M, Yauch RL, Lonergan KM, Whaley JM, Stemmer-Rachamimov AO, Louis DN et al. (1998). The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell 1: 959–968.
Park DM, Zhuang Z, Chen L, Szerlip N, Maric I, Li J et al. (2007). von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med 4: e60.
Pause A, Lee S, Worrell RA, Chen DY, Burgess WH, Linehan WM et al. (1997). The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci USA 94: 2156–2161.
Pfander D, Kobayashi T, Knight MC, Zelzer E, Chan DA, Olsen BR et al. (2004). Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development 131: 2497–2508.
Rankin EB, Tomaszewski JE, Haase VH . (2006). Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583.
Rorth P . (2002). Initiating and guiding migration: lessons from border cells. Trends Cell Biol 12: 325–331.
Schermer B, Ghenoiu C, Bartram M, Muller RU, Kotsis F, Hohne M et al. (2006). The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol 175: 547–554.
Shen C, Nettleton D, Jiang M, Kim SK, Powell-Coffman JA . (2005). Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J Biol Chem 280: 20580–20588.
Shen HC, Adem A, Ylaya K, Wilson A, He M, Lorang D et al. (2009). Deciphering von Hippel-Lindau (VHL/Vhl)-associated pancreatic manifestations by inactivating Vhl in specific pancreatic cell populations. PLoS One 4: e4897.
Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL et al. (2006). Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med 3: e290.
Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W . (2003). Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 425: 307–311.
Stebbins CE, Kaelin Jr WG, Pavletich NP . (1999). Structure of the VHL-ElonginC–ElonginB complex: implications for VHL tumor suppressor function. Science 284: 455–461.
Stenmark KR, Fagan KA, Frid MG . (2006). Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691.
Suzuki A, Ohno S . (2006). The PAR-aPKC system: lessons in polarity. J Cell Sci 119: 979–987.
Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y et al. (2010). Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7: 391–402.
Tang N, Mack F, Haase VH, Simon MC, Johnson RS . (2006). pVHL function is essential for endothelial extracellular matrix deposition. Mol Cell Biol 26: 2519–2530.
Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W . (2007). pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol 9: 588–595.
Thoma CR, Toso A, Gutbrodt KL, Reggi SP, Frew IJ, Schraml P et al. (2009). VHL loss causes spindle misorientation and chromosome instability. Nat Cell Biol 11: 994–1001.
Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B et al. (2006). Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med 12: 122–127.
Tsarouhas V, Senti KA, Jayaram SA, Tiklova K, Hemphala J, Adler J et al. (2007). Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell 13: 214–225.
Uv A, Cantera R, Samakovlis C . (2003). Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol 13: 301–309.
van der Velden YU, Wang L, Zevenhoven J, van Rooijen E, van Lohuizen M, Giles RH et al. (2011). The serine-threonine kinase LKB1 is essential for survival under energetic stress in zebrafish. Proc Natl Acad Sci USA 108: 4358–4363.
van Rooijen E, Voest EE, Logister I, Bussmann J, Korving J, van Eeden FJ et al. (2010). von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis Model Mech 3: 343–353.
van Rooijen E, Voest EE, Logister I, Korving J, Schwerte T, Schulte-Merker S et al. (2009). Zebrafish mutants in the von Hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood 113: 6449–6460.
Vortmeyer AO, Gnarra JR, Emmert-Buck MR, Katz D, Linehan WM, Oldfield EH et al. (1997). von Hippel-Lindau gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel-Lindau disease. Hum Pathol 28: 540–543.
Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL et al. (2009). Regulation of endocytosis via the oxygen-sensing pathway. Nat Med 15: 319–324.
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA et al. (2006). Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 5: 835–844.
Wingrove JA, O'Farrell PH . (1999). Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell 98: 105–114.
Woodward ER, Buchberger A, Clifford SC, Hurst LD, Affara NA, Maher ER . (2000). Comparative sequence analysis of the VHL tumor suppressor gene. Genomics 65: 253–265.
Woolworth JA, Nallamothu G, Hsu T . (2009). The Drosophila metastasis suppressor gene Nm23 homolog, awd, regulates epithelial integrity during oogenesis. Mol Cell Biol 29: 4679–4690.
Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T et al. (2003). Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol 13: 734–743.
Yang H, Minamishima YA, Yan Q, Schlisio S, Ebert BL, Zhang X et al. (2007). pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol Cell 28: 15–27.
Yaqoob N, Schwerte T . (2010). Cardiovascular and respiratory developmental plasticity under oxygen depleted environment and in genetically hypoxic zebrafish (Danio rerio). Comp Biochem Physiol A Mol Integr Physiol 156: 475–484.
Young AP, Schlisio S, Minamishima YA, Zhang Q, Li L, Grisanzio C et al. (2008). VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol 10: 361–369.
Zehetner J, Danzer C, Collins S, Eckhardt K, Gerber PA, Ballschmieter P et al. (2008). PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic beta cells. Genes Dev 22: 3135–3146.
Zlotogora J . (1994). High frequencies of human genetic diseases: founder effect with genetic drift or selection? Am J Med Genet 49: 10–13.
Acknowledgements
This work was funded by the National Cancer Institute (USA) grant (RO1CA109860).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hsu, T. Complex cellular functions of the von Hippel–Lindau tumor suppressor gene: insights from model organisms. Oncogene 31, 2247–2257 (2012). https://doi.org/10.1038/onc.2011.442
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.442
Keywords
This article is cited by
-
Tumor heterogeneity in VHL drives metastasis in clear cell renal cell carcinoma
Signal Transduction and Targeted Therapy (2023)
-
Genetic kidney diseases: Caenorhabditis elegans as model system
Cell and Tissue Research (2017)
-
Role of primary cilia in non-dividing and post-mitotic cells
Cell and Tissue Research (2017)
-
Inactivation of the tumor suppressor gene von Hippel-Lindau (VHL) in granulocytes contributes to development of liver hemangiomas in a mouse model
BMC Cancer (2016)
-
The von Hippel–Lindau tumor suppressor regulates programmed cell death 5-mediated degradation of Mdm2
Oncogene (2015)