Abstract
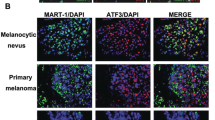
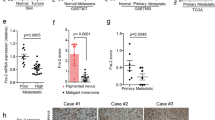
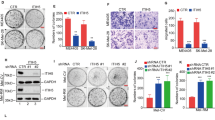
Nuclear factor-κB (NF-κB) inducing kinase (NIK) is a MAP3K that regulates the activation of NF-κB. NIK is often highly expressed in tumor cells, including melanoma, but the significance of this in melanoma progression has been unclear. Tissue microarray analysis of NIK expression reveals that dysplastic nevi (n=22), primary (n=15) and metastatic melanoma (n=13) lesions showed a statistically significant elevation in NIK expression when compared with benign nevi (n=30). Moreover, when short hairpin RNA techniques were used to knock-down NIK, the resultant NIK-depleted melanoma cell lines exhibited decreased proliferation, increased apoptosis, delayed cell cycle progression and reduced tumor growth in a mouse xenograft model. As expected, when NIK was depleted there was decreased activation of the non-canonical NF-κB pathway, whereas canonical NF-κB activation remained intact. NIK depletion also resulted in reduced expression of genes that contribute to tumor growth, including CXCR4, c-MYC and c-MET, and pro-survival factors such as BCL2 and survivin. These changes in gene expression are not fully explained by the attenuation of the non-canonical NF-κB pathway. Shown here for the first time is the demonstration that NIK modulates β-catenin-mediated transcription to promote expression of survivin. NIK-depleted melanoma cells exhibited downregulation of survivin as well as other β-catenin regulated genes including c-MYC, c-MET and CCND2. These data indicate that NIK mediates both β-catenin and NF-κB regulated transcription to modulate melanoma survival and growth. Thus, NIK may be a promising therapeutic target for melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F et al. (2007). Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12: 115–130.
Baldwin AS . (2001). Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest 107: 241–246.
Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A . (1991). Effects of MGSA/GRO alpha on melanocyte transformation. Oncogene 6: 1115–1124.
Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST . (2002). Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res 62: 5126–5128.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J et al. (2011). Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516.
Chariot A . (2009). The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol 19: 404–413.
Cheli Y, Ohanna M, Ballotti R, Bertolotto C . (2010). Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res 23: 27–40.
Chin L, Merlino G, DePinho RA . (1998). Malignant melanoma: modern black plague and genetic black box. Genes Dev 12: 3467–3481.
Cole AM, Myant K, Reed KR, Ridgway RA, Athineos D, Van den Brink GR et al. (2010). Cyclin D2-cyclin-dependent kinase 4/6 is required for efficient proliferation and tumorigenesis following Apc loss. Cancer Res 70: 8149–8158.
Conze DB, Zhao Y, Ashwell JD . (2010). Non-canonical NF-kappaB activation and abnormal B cell accumulation in mice expressing ubiquitin protein ligase-inactive c-IAP2. PLoS Biol 8: e1000518.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S et al. (2002). Mutations of the BRAF gene in human cancer. Nature 417: 949–954.
Delmas V, Beermann F, Martinozzi S, Carreira S, Ackermann J, Kumasaka M et al. (2007). Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev 21: 2923–2935.
Demchenko YN, Glebov OK, Zingone A, Keats JJ, Bergsagel PL, Kuehl WM . (2010). Classical and/or alternative NF-kappaB pathway activation in multiple myeloma. Blood 115: 3541–3552.
Devalaraja MN, Wang DZ, Ballard DW, Richmond A . (1999). Elevated constitutive IkappaB kinase activity and IkappaB-alpha phosphorylation in Hs294T melanoma cells lead to increased basal MGSA/GRO-alpha transcription. Cancer Res 59: 1372–1377.
Dhawan P, Richmond A . (2002). A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J Biol Chem 277: 7920–7928.
Dhomen N, Marais R . (2009). BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am 23: 529–545, ix.
Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I et al. (2008). CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature 455: 547–551.
Grossman D, Kim PJ, Schechner JS, Altieri DC . (2001). Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc Natl Acad Sci U S A 98: 635–640.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT et al. (1998). Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512.
Hilmi C, Larribere L, Giuliano S, Bille K, Ortonne JP, Ballotti R et al. (2008). IGF1 promotes resistance to apoptosis in melanoma cells through an increased expression of BCL2, BCL-X(L), and survivin. J Invest Dermatol 128: 1499–1505.
Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ et al. (2007). Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell 12: 131–144.
Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC . (2003). Survivin and molecular pathogenesis of colorectal cancer. Lancet 362: 205–209.
Lai AZ, Abella JV, Park M . (2009). Crosstalk in Met receptor oncogenesis. Trends Cell Biol 19: 542–551.
Lecis D, Drago C, Manzoni L, Seneci P, Scolastico C, Mastrangelo E et al. (2010). Novel SMAC-mimetics synergistically stimulate melanoma cell death in combination with TRAIL and Bortezomib. Br J Cancer 102: 1707–1716.
Lev DC, Ruiz M, Mills L, McGary EC, Price JE, Bar-Eli M . (2003). Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Mol Cancer Ther 2: 753–763.
Ling L, Cao Z, Goeddel DV . (1998). NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci U S A 95: 3792–3797.
Liston P, Fong WG, Kelly NL, Toji S, Miyazaki T, Conte D et al. (2001). Identification of XAF1 as an antagonist of XIAP anti-Caspase activity. Nat Cell Biol 3: 128–133.
Malbon CC . (2005). Beta-catenin, cancer, and G proteins: not just for frizzleds anymore. Sci STKE 2005: pe35.
Malinin NL, Boldin MP, Kovalenko AV, Wallach D . (1997). MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature 385: 540–544.
McGary EC, Lev DC, Bar-Eli M . (2002). Cellular adhesion pathways and metastatic potential of human melanoma. Cancer Biol Ther 1: 459–465.
McGill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK et al. (2002). Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 109: 707–718.
Moore SR, Persons DL, Sosman JA, Bobadilla D, Bedell V, Smith DD et al. (2008). Detection of copy number alterations in metastatic melanoma by a DNA fluorescence in situ hybridization probe panel and array comparative genomic hybridization: a southwest oncology group study (S9431). Clin Cancer Res 14: 2927–2935.
Neely RJ, Brose MS, Gray CM, McCorkell KA, Leibowitz JM, Ma C et al. (2010). The RET/PTC3 oncogene activates classical NF-kappaB by stabilizing NIK. Oncogene 30: 87–96.
Ng KC, Campos EI, Martinka M, Li G . (2004). XAF1 expression is significantly reduced in human melanoma. J Invest Dermatol 123: 1127–1134.
Pastorino F, Brignole C, Marimpietri D, Pagnan G, Morando A, Ribatti D et al. (2003). Targeted liposomal c-myc antisense oligodeoxynucleotides induce apoptosis and inhibit tumor growth and metastases in human melanoma models. Clin Cancer Res 9: 4595–4605.
Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J et al. (2007). Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 12: 445–456.
Plenchette S, Cheung HH, Fong WG, LaCasse EC, Korneluk RG . (2007). The role of XAF1 in cancer. Curr Opin Investig Drugs 8: 469–476.
Puri N, Ahmed S, Janamanchi V, Tretiakova M, Zumba O, Krausz T et al. (2007). c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res 13: 2246–2253.
Puzanov I, Flaherty KT . (2010). Targeted molecular therapy in melanoma. Semin Cutan Med Surg 29: 196–201.
Rosebeck S, Madden L, Jin X, Gu S, Apel IJ, Appert A et al. (2011). Cleavage of NIK by the API2-MALT1 fusion oncoprotein leads to noncanonical NF-kappaB activation. Science 331: 468–472.
Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P . (1997). Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science 275: 1790–1792.
Ruggero D . (2009). The role of Myc-induced protein synthesis in cancer. Cancer Res 69: 8839–8843.
Saitoh Y, Martinez Bruyn VJ, Uota S, Hasegawa A, Yamamoto N, Imoto I et al. (2010). Overexpression of NF-kappaB inducing kinase underlies constitutive NF-kappaB activation in lung cancer cells. Lung Cancer 70: 263–270.
Saitoh Y, Yamamoto N, Dewan MZ, Sugimoto H, Martinez BV, Iwasaki Y et al. (2008). Overexpressed NF-\{kappa\}B inducing kinase contributes to the tumorigenesis of adult T-cell leukemia and Ho. Blood 111: 5118–5129.
Scala S, Giuliano P, Ascierto PA, Ierano C, Franco R, Napolitano M et al. (2006). Human melanoma metastases express functional CXCR4. Clin Cancer Res 12: 2427–2433.
Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P et al. (2005). Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res 11: 1835–1841.
Sinnberg T, Menzel M, Kaesler S, Biedermann T, Sauer B, Nahnsen S et al. (2010). Suppression of casein kinase 1alpha in melanoma cells induces a switch in beta-catenin signaling to promote metastasis. Cancer Res 70: 6999–7009.
Smith C, Andreakos E, Crawley JB, Brennan FM, Feldmann M, Foxwell BM . (2001). NF-kappaB-inducing kinase is dispensable for activation of NF-kappaB in inflammatory settings but essential for lymphotoxin beta receptor activation of NF-kappaB in primary human fibroblasts. J Immunol 167: 5895–5903.
Sullivan RJ, Atkins MB . (2009). Molecular-targeted therapy in malignant melanoma. Expert rev anticancer ther 9: 567–581.
Van Antwerp DJ, Martin SJ, Verma IM, Green DR . (1998). Inhibition of TNF-induced apoptosis by NF-kappa B. Trends Cell Biol 8: 107–111.
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al. (2007). IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 131: 669–681.
Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU et al. (2007). IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 131: 682–693.
Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S et al. (2009). Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet 41: 843–848.
Wang CY, Mayo MW, Baldwin Jr AS . (1996). TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science 274: 784–787.
Wang D, Yang W, Du J, Devalaraja MN, Liang P, Matsumoto K et al. (2000). MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression. Oncogene 19: 4647–4659.
Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A . (2006). BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin Cancer Res 12: 950–960.
Yang J, Pan WH, Clawson GA, Richmond A . (2007). Systemic targeting inhibitor of kappaB kinase inhibits melanoma tumor growth. Cancer Res 67: 3127–3134.
Yang J, Richmond A . (2001). Constitutive IkappaB kinase activity correlates with nuclear factor-kappaB activation in human melanoma cells. Cancer Res 61: 4901–4909.
Yang J, Splittgerber R, Yull FE, Kantrow S, Ayers GD, Karin M et al. (2010). Conditional ablation of Ikkb inhibits melanoma tumor development in mice. J Clin Invest 120: 2563–2574.
Yin L, Wu L, Wesche H, Arthur CD, White JM, Goeddel DV et al. (2001). Defective lymphotoxin-beta receptor-induced NF-kappaB transcriptional activity in NIK-deficient mice. Science 291: 2162–2165.
Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ et al. (2001). Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res 61: 8664–8667.
Acknowledgements
We would like to thank Dr Robert Schreiber (Washington University) for generously sharing Nik−/− MEFs, and Vanderbilt Functional Genomic Shared Resource Core, VA Flow Cytometry Core and Vanderbilt Immunohistochemistry Core for technical help. We thank Dr Joseph T Roland (Epithelial Biology Center) for assistance with the ariol scanning and quantitation of TMAs. We thank Dr Mark Kelly and Vanderbilt melanoma group for providing melanoma tissues and Dr Sara Kantrow for reviewing some of the TMA slides. We thank Dr Fiona Yull and Dr Punita Dhawan for thoughtful discussion and Vanderbilt Editor Club for careful reading of the manuscript. Grant Support: This work is supported by NIH, CA 098807-01 and the Department of Veterans Affairs, Career Scientist Award and Merit Award (to AR).
Author contributions: Designed experiments and wrote paper: YMT and AR. Performed experiments: YMT, YS, JY and RS. Analyzed data: YMT, JY and RS. Shared reagents: SN. Contributed human tissue samples for TMA: AB, CS and CM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Thu, Y., Su, Y., Yang, J. et al. NF-κB inducing kinase (NIK) modulates melanoma tumorigenesis by regulating expression of pro-survival factors through the β-catenin pathway. Oncogene 31, 2580–2592 (2012). https://doi.org/10.1038/onc.2011.427
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.427