Abstract
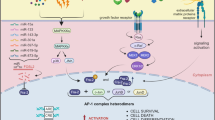
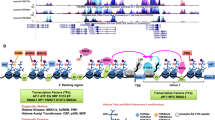
Fos-related antigen-1 (Fra-1) is a member of the Activator Protein-1 (AP-1) transcription factor superfamily that is overexpressed in a variety of cancers, including colon, breast, lung, bladder and brain. High Fra-1 levels are associated with enhanced cell proliferation, survival, migration and invasion. Despite its frequent overexpression, the molecular mechanisms that regulate the accumulation of Fra-1 proteins in tumour cells are not well understood. Here, we show that turnover of Fra-1, which does not require ubiquitylation, is cooperatively regulated by two distinct mechanisms—association with the 19S proteasomal subunit, TBP-1, and by a C-terminal degron, which acts independently of TBP-1, but is regulated by RAS–ERK (extracellular signal-regulated kinase) signalling. TBP-1 depletion stabilized Fra-1 and further increased its levels in tumour cells expressing RAS–ERK pathway oncogenes. These effects correlated with increased AP-1 transcriptional activity. We suggest that during Fra-1 degradation, association with TBP-1 provides a mechanism for ubiquitin-independent proteasomal recognition, while the C terminus of the protein regulates its subsequent proteolytic processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Acquaviva C, Brockly F, Ferrara P, Bossis G, Salvat C, Jariel-Encontre I et al. (2001). Identification of a C-terminal tripeptide motif involved in the control of rapid proteasomal degradation of c-Fos proto-oncoprotein during the G(0)-to-S phase transition. Oncogene 20: 7563–7572.
Adiseshaiah P, Lindner DJ, Kalvakolanu DV, Reddy SP . (2007). FRA-1 proto-oncogene induces lung epithelial cell invasion and anchorage-independent growth in vitro, but is insufficient to promote tumor growth in vivo. Cancer Res 67: 6204–6211.
Andreolas C, Kalogeropoulou M, Voulgari A, Pintzas A . (2008). Fra-1 regulates vimentin during Ha-RAS-induced epithelial mesenchymal transition in human colon carcinoma cells. Int J Cancer 122: 1745–1756.
Asher G, Tsvetkov P, Kahana C, Shaul Y . (2005). A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73. Genes Dev 19: 316–321.
Basbous J, Chalbos D, Hipskind R, Jariel-Encontre I, Piechaczyk M . (2007). Ubiquitin-independent proteasomal degradation of Fra-1 is antagonized by Erk1/2 pathway-mediated phosphorylation of a unique C-terminal destabilizer. Mol Cell Biol 27: 3936–3950.
Basbous J, Jariel-Encontre I, Gomard T, Bossis G, Piechaczyk M . (2008). Ubiquitin-independent- versus ubiquitin-dependent proteasomal degradation of the c-Fos and Fra-1 transcription factors: is there a unique answer? Biochimie 90: 296–305.
Baugh JM, Viktorova EG, Pilipenko EV . (2009). Proteasomes can degrade a significant proportion of cellular proteins independent of ubiquitination. J Mol Biol 386: 814–827.
Belguise K, Kersual N, Galtier F, Chalbos D . (2005). FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene 24: 1434–1444.
Burch PM, Yuan Z, Loonen A, Heintz NH . (2004). An extracellular signal-regulated kinase 1- and 2-dependent program of chromatin trafficking of c-Fos and Fra-1 is required for cyclin D1 expression during cell cycle reentry. Mol Cell Biol 24: 4696–4709.
Casalino L, Bakiri L, Talotta F, Weitzman JB, Fusco A, Yaniv M et al. (2007). Fra-1 promotes growth and survival in RAS-transformed thyroid cells by controlling cyclin A transcription. EMBO J 26: 1878–1890.
Casalino L, De Cesare D, Verde P . (2003). Accumulation of Fra-1 in ras-transformed cells depends on both transcriptional autoregulation and MEK-dependent posttranslational stabilization. Mol Cell Biol 23: 4401–4415.
Chen C, Zhou Z, Guo P, Dong JT . (2007a). Proteasomal degradation of the KLF5 transcription factor through a ubiquitin-independent pathway. FEBS Lett 581: 1124–1130.
Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM . (2007b). Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol Cell 26: 843–852.
Chinenov Y, Kerppola TK . (2001). Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20: 2438–2452.
Cohn MA, Hjelmso I, Wu LC, Guldberg P, Lukanidin EM, Tulchinsky EM . (2001). Characterization of Sp1, AP-1, CBF and KRC binding sites and minisatellite DNA as functional elements of the metastasis-associated mts1/S100A4 gene intronic enhancer. Nucleic Acids Res 29: 3335–3346.
Corn PG, McDonald III ER, Herman JG, El-Deiry WS . (2003). Tat-binding protein-1, a component of the 26S proteasome, contributes to the E3 ubiquitin ligase function of the von Hippel-Lindau protein. Nat Genet 35: 229–237.
Debinski W, Gibo DM . (2005). Fos-related antigen 1 modulates malignant features of glioma cells. Mol Cancer Res 3: 237–249.
Doehn U, Hauge C, Frank SR, Jensen CJ, Duda K, Nielsen JV et al. (2009). RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Mol Cell 35: 511–522.
Dong Z, Xu RH, Kim J, Zhan SN, Ma WY, Colburn NH et al. (1996). AP-1/jun is required for early Xenopus development and mediates mesoderm induction by fibroblast growth factor but not by activin. J Biol Chem 271: 9942–9946.
Finley D . (2009). Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78: 477–513.
Gallastegui N, Groll M . (2010). The 26S proteasome: assembly and function of a destructive machine. Trends Biochem Sci 35: 634–642.
Gomard T, Jariel-Encontre I, Basbous J, Bossis G, Mocquet-Torcy G, Piechaczyk M . (2008). Fos family protein degradation by the proteasome. Biochem Soc Trans 36: 858–863.
Higashitsuji H, Itoh K, Nagao T, Dawson S, Nonoguchi K, Kido T et al. (2000). Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat Med 6: 96–99.
Hoyt MA, Coffino P . (2004). Ubiquitin-free routes into the proteasome. Cell Mol Life Sci 61: 1596–1600.
Ishizuka T, Satoh T, Monden T, Shibusawa N, Hashida T, Yamada M et al. (2001). Human immunodeficiency virus type 1 Tat binding protein-1 is a transcriptional coactivator specific for TR. Mol Endocrinol 15: 1329–1343.
Jariel-Encontre I, Bossis G, Piechaczyk M . (2008). Ubiquitin-independent degradation of proteins by the proteasome. Biochim Biophys Acta 1786: 153–177.
Kakumoto K, Sasai K, Sukezane T, Oneyama C, Ishimaru S, Shibutani K et al. (2006). FRA1 is a determinant for the difference in RAS-induced transformation between human and rat fibroblasts. Proc Natl Acad Sci USA 103: 5490–5495.
Kalejta RF, Shenk T . (2003). Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proc Natl Acad Sci USA 100: 3263–3268.
Karin M, Liu Z, Zandi E . (1997). AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246.
Kustikova O, Kramerov D, Grigorian M, Berezin V, Bock E, Lukanidin E et al. (1998). Fra-1 induces morphological transformation and increases in vitro invasiveness and motility of epithelioid adenocarcinoma cells. Mol Cell Biol 18: 7095–7105.
Lam YA, Lawson TG, Velayutham M, Zweier JL, Pickart CM . (2002). A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature 416: 763–767.
Luo YP, Zhou H, Krueger J, Kaplan C, Liao D, Markowitz D et al. (2010). The role of proto-oncogene Fra-1 in remodeling the tumor microenvironment in support of breast tumor cell invasion and progression. Oncogene 29: 662–673.
Park BW, O′Rourke DM, Wang Q, Davis JG, Post A, Qian X et al. (1999). Induction of the Tat-binding protein 1 gene accompanies the disabling of oncogenic erbB receptor tyrosine kinases. Proc Natl Acad Sci USA 96: 6434–6438.
Pollice A, Sepe M, Villella VR, Tolino F, Vivo M, Calabro V et al. (2007). TBP-1 protects the human oncosuppressor p14ARF from proteasomal degradation. Oncogene 26: 5154–5162.
Pollock CB, Shirasawa S, Sasazuki T, Kolch W, Dhillon AS . (2005). Oncogenic K-RAS is required to maintain changes in cytoskeletal organization, adhesion, and motility in colon cancer cells. Cancer Res 65: 1244–1250.
Prakash S, Tian L, Ratliff KS, Lehotzky RE, Matouschek A . (2004). An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat Struct Mol Biol 11: 830–837.
Ramos-Nino ME, Scapoli L, Martinelli M, Land S, Mossman BT . (2003). Microarray analysis and RNA silencing link fra-1 to cd44 and c-met expression in mesothelioma. Cancer Res 63: 3539–3545.
Saksela K, Baltimore D . (1993). Negative regulation of immunoglobulin kappa light-chain gene transcription by a short sequence homologous to the murine B1 repetitive element. Mol Cell Biol 13: 3698–3705.
Satoh T, Ishizuka T, Tomaru T, Yoshino S, Nakajima Y, Hashimoto K et al. (2009). Tat-binding protein-1 (TBP-1), an ATPase of 19S regulatory particles of the 26S proteasome, enhances androgen receptor function in cooperation with TBP-1-interacting protein/Hop2. Endocrinology 150: 3283–3290.
Shin S, Dimitri CA, Yoon SO, Dowdle W, Blenis J . (2010). but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell 38: 114–127.
Takeuchi J, Chen H, Coffino P . (2007). Proteasome substrate degradation requires association plus extended peptide. EMBO J 26: 123–131.
Talotta F, Mega T, Bossis G, Casalino L, Basbous J, Jariel-Encontre I et al. (2003). Heterodimerization with Fra-1 cooperates with the ERK pathway to stabilize c-Jun in response to the RAS oncoprotein. Oncogene 29: 4732–4740.
Terasawa K, Okazaki K, Nishida E . (2003). Regulation of c-Fos and Fra-1 by the MEK5-ERK5 pathway. Genes Cells 8: 263–273.
Vial E, Marshall CJ . (2003). Elevated ERK-MAP kinase activity protects the FOS family member FRA-1 against proteasomal degradation in colon carcinoma cells. J Cell Sci 116: 4957–4963.
Vial E, Sahai E, Marshall CJ . (2003). ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 4: 67–79.
Vikhanskaya F, Toh WH, Dulloo I, Wu Q, Boominathan L, Ng HH et al. (2007). p73 supports cellular growth through c-Jun-dependent AP-1 transactivation. Nat Cell Biol 9: 698–705.
von Kriegsheim A, Pitt A, Grindlay GJ, Kolch W, Dhillon AS . (2006). Regulation of the Raf-MEK-ERK pathway by protein phosphatase 5. Nat Cell Biol 8: 1011–1016.
Young MR, Colburn NH . (2006). Fra-1 a target for cancer prevention or intervention. Gene 379: 1–11.
Young MR, Nair R, Bucheimer N, Tulsian P, Brown N, Chapp C et al. (2002). Transactivation of Fra-1 and consequent activation of AP-1 occur extracellular signal-regulated kinase dependently. Mol Cell Biol 22: 587–598.
Zhang M, Pickart CM, Coffino P . (2003). Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. Embo J 22: 1488–1496.
Acknowledgements
We thank Drs Nishida, Satoh, Greer and Gillespie for generously providing reagents used in this study, and Dr Willie Bienvenut for assistance with the mass spectrometry experiments. This work was supported by grants from the National Health and Medical Research Council of Australia (to ASD and RDH) and the Association for International Cancer Research (to ET).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Pakay, J., Diesch, J., Gilan, O. et al. A 19S proteasomal subunit cooperates with an ERK MAPK-regulated degron to regulate accumulation of Fra-1 in tumour cells. Oncogene 31, 1817–1824 (2012). https://doi.org/10.1038/onc.2011.375
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.375
Keywords
This article is cited by
-
ERCC1 abundance is an indicator of DNA repair-apoptosis decision upon DNA damage
Cell Death Discovery (2024)
-
Expression and function of FRA1 protein in tumors
Molecular Biology Reports (2020)
-
The inflammatory cytokine IL-6 induces FRA1 deacetylation promoting colorectal cancer stem-like properties
Oncogene (2019)
-
PR55α-containing protein phosphatase 2A complexes promote cancer cell migration and invasion through regulation of AP-1 transcriptional activity
Oncogene (2015)
-
FRA-1 as a driver of tumour heterogeneity: a nexus between oncogenes and embryonic signalling pathways in cancer
Oncogene (2015)