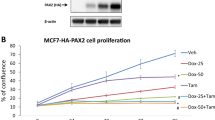
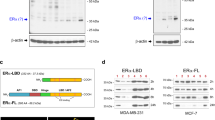
Abstract
Recognition of the pivotal role of estrogen in the aetiology of breast cancer has led to the development of antiestrogens (AE), such as tamoxifen (TAM) as effective therapies for the treatment and prevention of this disease. However, despite their widespread clinical efficacy, response to AEs is often short-lived, and acquired or innate therapeutic resistance remains a major obstacle in the successful treatment of breast cancer. Thus, delineating the intracellular pathways that mediate the cellular response to estrogen could potentially lead to new, more effective approaches to the treatment of breast cancer, particularly endocrine-resistant disease. Here, we have identified the BCL-2 homology 3 (BH3)-only, pro-apoptotic regulator, PUMA (p53 upregulated modulator of apoptosis) as an estrogen target gene that is acutely downregulated in response to estrogen in breast cancer cell lines, independently of their p53 status. PUMA is transcriptionally upregulated following treatment with TAM, and knock down of PUMA expression in these cells attenuates the apoptotic response to TAM. Furthermore, low PUMA expression in breast carcinomas is significantly associated with breast cancer-specific death (P=0.0014 and P=0.0115, for mRNA and protein, respectively), and worse outcome in TAM-treated patients (mRNA, P=1.49e-05). These findings suggest that the dysregulation of apoptotic signaling pathways such as those executed through PUMA, can significantly impact on both the progression and therapeutic responsiveness of breast cancer. Moreover, they provide a convincing rationale for exploring new therapeutic approaches involving endocrine and non-endocrine therapies that target apoptotic pathways as an effective strategy for tackling endocrine refractory disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- AE:
-
anti-estrogen
- TAM:
-
tamoxifen
- FCS:
-
fetal calf serum
- 4-OHT:
-
4-hydroxytamoxifen
- ER:
-
estrogen receptor
- ChIP:
-
chromatin immunoprecipitation
- TMA:
-
tissue microarray
- PR:
-
progesterone receptor
- EGFR:
-
epidermal growth factor receptor
- ERE:
-
estrogen response element
- HER2:
-
human epidermal growth factor receptor 2
References
Anderson LR, Sutherland RL, Butt AJ . (2010). BAG-1 overexpression attenuates luminal apoptosis in MCF-10A mammary epithelial cells through enhanced RAF-1 activation. Oncogene 29: 527–538.
Butt AJ, Caldon CE, McNeil CM, Swarbrick A, Musgrove EA, Sutherland RL . (2008). Cell cycle machinery: links with genesis and treatment of breast cancer. Adv Exp Med Biol 630: 189–205.
Butt AJ, McNeil CM, Musgrove EA, Sutherland RL . (2005). Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E. Endocr Rel Cancer 12: S47–S59.
Butt AJ, Musgrove EA, Sutherland RL . (2007). Live or let die: oestrogen regulation of survival signalling in endocrine response. Breast Cancer Res 9: 306.
Butt AJ, Roberts CG, Seawright AA, Oelrichs PB, MacLeod JK, Liaw TYE et al. (2006). A novel plant toxin, persin, with in vivo activity in the mammary gland, induces Bim-dependent apoptosis in human breast cancer cells. Mol Cancer Ther 5: 2300–2309.
Cannings E, Kirkegaard T, Tovey SM, Dunne B, Cooke TG, Bartlett JMS . (2007). Bad expression predicts outcome in patients treated with tamoxifen. Breast Cancer Res Treat 102: 173–179.
Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J et al. (2006). Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38: 1289–1297.
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG et al. (2005). Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403.
Ellis MJ, Rosen E, Dressman H, Marks J . (2003). Neoadjuvant comparisons of aromatase inhibitors and tamoxifen: pretreatment determinants of response and on-treatment effect. J Steroid Biochem Mol Biol 86: 301–307.
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H et al. (2008). Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14: 18–27.
Frasor J, Danes JM, Komm B, Chang KCN, Lyttle CR, Katzenellenbogen BS . (2003). Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144: 4562–4574.
Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE et al. (2008). Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol 28: 5391–5402.
Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q et al. (2009). An online survival analysis tool to rapidly assess the effect of 22 277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res Treat 123: 725–731.
Hammerich-Hille S, Kaipparettu BA, Tsimelzon A, Creighton CJ, Jiang S, Polo JM et al. (2010). SAFB1 mediates repression of immune regulators and apoptotic genes in breast cancer cells. J Biol Chem 285: 3608–3616.
Han J, Flemington C, Houghton AB, Gu Z, Zambettidagger GP, Lutz RJ et al. (2001). Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci USA 98: 11318–11323.
Hanahan D, Weinberg RA . (2000). The hallmarks of cancer. Cell 200: 57–70.
Hur J, Chesnes J, Coser KR, Lee RS, Geck P, Isselbacher KJ et al. (2004). The Bik BH3-only protein is induced in estrogen-starved and antiestrogen-exposed breast cancer cells and provokes apoptosis. Proc Natl Acad Sci USA 101: 2351–2356.
Hurvitz SA, Pietras RJ . (2008). Rational management of endocrine resistance in breast cancer: a comprehensive review of estrogen receptor biology, treatment options, and future directions. Cancer 113: 2385–2397.
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J et al. (2003). Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Res 52: 5204–5207.
Jordan VC . (2009). A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res 69: 1243–1254.
Karst AM, Dai DL, Martinka M, Li G . (2005). PUMA expression is significantly reduced in human cutaneous melanomas. Oncogene 24: 1111–1116.
Millar EKA, Anderson LR, McNeil CM, O'Toole SA, Pinese M, Crea P et al. (2008). BAG-1 predicts patient outcome and tamoxifen responsiveness in ER-positive invasive ductal carcinoma of the breast. Br J Cancer 100: 123–133.
Musgrove EA, Sergio CM, Loi S, Inman CK, Anderson LR, Alles MC et al. (2008). Identification of functional networks of estrogen- and c-Myc-responsive genes and their relationship to response to tamoxifen therapy in breast cancer. PLoS One 3: e2987.
Musgrove EA, Sutherland RL . (2009). Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 9: 631–643.
Nakano K, Vousden KH . (2001). PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7: 683–694.
Naresh A, Thor AD, Edgerton SM, Torkko KC, Kumar R, Jones FE . (2008). The HER4/4ICD estrogen receptor coactivator and BH3-only protein is an effector of tamoxifen-induced apoptosis. Cancer Res 68: 6387–6395.
Perillo B, Sasso A, Abbondanza C, Palumbo G . (2000). 17b-estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol 20: 2890–2901.
Reimer T, Koczan D, Muller H, Friese K, Thiesen H-J, Gerber B . (2002). Tumour Fas ligand:Fas ratio greater than 1 is an independent marker of relative resistance to tamoxifen therapy in hormone receptor positive breast cancer. Breast Cancer Res 4: R9.
Runnebaum IB, Nagarajan M, Bowman M, Soto D, Sukumar S . (1991). Mutations in p53 as potential molecular markers for human breast cancer. Proc Natl Acad Sci USA 88: 10657–10661.
Swaby RF, Sharma CG, Jordan VC . (2007). SERMs for the treatment and prevention of breast cancer. Rev Endocr Metab Disord 8: 229–239.
van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW et al. (2002). A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347: 1999–2009.
Vousden KH . (2005). p53 and PUMA: a deadly duo. Science 309: 1685–1686.
Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L . (2003). PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA 100: 1931–1936.
Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B . (2001). PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7: 673–682.
Yu J, Zhang L . (2005). The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 331: 851–858.
Yu J, Zhang L . (2009). PUMA, a potent killer with or without p53. Oncogene 27: S71–S83.
Acknowledgements
This research was supported by a National Health and Medical Research Council (NHMRC) of Australia Program Grant 535903, the Australian Cancer Research Fund, the RT Hall Trust, the Petre Foundation, a Cancer Institute NSW Career Development and Support Fellowship (AJB), Cancer Institute NSW Clinical Research Fellowships (EKAM and SAO’T) and an NHMRC health professional training fellowship (SAO’T).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Roberts, C., Millar, E., O'Toole, S. et al. Identification of PUMA as an estrogen target gene that mediates the apoptotic response to tamoxifen in human breast cancer cells and predicts patient outcome and tamoxifen responsiveness in breast cancer. Oncogene 30, 3186–3197 (2011). https://doi.org/10.1038/onc.2011.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.36
Keywords
This article is cited by
-
Estrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer
Oncogene (2014)
-
Tranilast enhances the anti-tumor effects of tamoxifen on human breast cancer cells in vitro
Journal of Biomedical Science (2013)
-
Insulin-like growth factor 1 attenuates antiestrogen- and antiprogestin-induced apoptosis in ER+ breast cancer cells by MEK1 regulation of the BH3-only pro-apoptotic protein Bim
Breast Cancer Research (2012)
-
Hormonal Resistance in Breast Cancer: Evolving Treatment Strategies
Current Breast Cancer Reports (2012)