Abstract
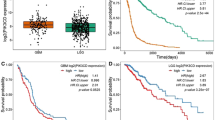
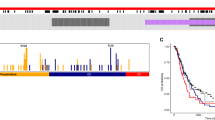
The phosphatidylinositol-3-OH kinase (PI3K)-Akt pathway is activated in cancer by genetic or epigenetic events and efforts are under way to develop targeted therapies. phosphatase and tensin homolog deleted on chromosome 10 (PTEN) tumor suppressor is the major brake of the pathway and a common target for inactivation in glioblastoma, one of the most aggressive and therapy-resistant cancers. To achieve potent inhibition of the PI3K-Akt pathway in glioblastoma, we need to understand its mechanism of activation by investigating the interplay between its regulators. We show here that PTEN modulates the PI3K-Akt pathway in glioblastoma within a tumor suppressor network that includes Na+/H+ exchanger regulatory factor 1 (NHERF1) and pleckstrin-homology domain leucine-rich repeat protein phosphatases 1 (PHLPP1). The NHERF1 adaptor, previously characterized by our group as a PTEN ligand and regulator, shows also PTEN-independent Akt-modulating effects that led us to identify the PHLPP1/PHLPP2 Akt phosphatases as NHERF1 ligands. NHERF1 interacts via its PDZ domains with PHLPP1/PHLPP2 and scaffolds heterotrimeric complexes with PTEN. Functionally, PHLPP1 requires NHERF1 for membrane localization and growth-suppressive effects. PHLPP1 loss boosts Akt phosphorylation only in PTEN-negative cells and cooperates with PTEN loss for tumor growth. In a panel of low-grade and high-grade glioma patient samples, we show for the first time a significant disruption of all three members of the PTEN-NHERF1-PHLPP1 tumor suppressor network in high-grade tumors, correlating with Akt activation and patient's abysmal survival. We thus propose a PTEN-NHERF1-PHLPP PI3K-Akt pathway inhibitory network that relies on molecular interactions and can undergo parallel synergistic hits in glioblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J . (1998). 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol 8: 69–81.
Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C et al. (2007). NCBI GEO: mining tens of millions of expression profiles—database and tools update. Nucleic Acids Res 35: D760–D765.
Brognard J, Newton AC . (2008). PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab 19: 223–230.
Brognard J, Sierecki E, Gao T, Newton AC . (2007). PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell 25: 917–931.
Cardone RA, Bellizzi A, Busco G, Weinman EJ, Dell'Aquila ME, Casavola V et al. (2007). The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor cells. Mol Biol Cell 18: 1768–1780.
Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF . (2008). The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets 8: 187–198.
Courtney KD, Corcoran RB, Engelman JA . (2010). The PI3K pathway as drug target in human cancer. J Clin Oncol 28: 1075–1083.
Fouassier L, Yun CC, Fitz JG, Doctor RB . (2000). Evidence for ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) self-association through PDZ-PDZ interactions. J Biol Chem 275: 25039–25045.
Franke TF . (2008). PI3K/Akt: getting it right matters. Oncogene 27: 6473–6488.
Gao T, Furnari F, Newton AC . (2005). PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell 18: 13–24.
Georgescu MM . (2010). PTEN tumor suppressor network in PI3K-Akt pathway control. Genes & Cancer 1: 1170–1177.
Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H . (1999). The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA 96: 10182–10187.
Georgescu MM, Morales FC, Molina JR, Hayashi Y . (2008). Roles of NHERF1/EBP50 in cancer. Curr Mol Med 8: 459–468.
Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ et al. (1998). A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA 95: 8496–8501.
Hayashi Y, Molina JR, Hamilton SR, Georgescu MM . (2010). NHERF1/EBP50 is a new marker in colorectal cancer. Neoplasia 12: 1013–1022.
Kreimann EL, Morales FC, de Orbeta-Cruz J, Takahashi Y, Adams H, Liu TJ et al. (2007). Cortical stabilization of beta-catenin contributes to NHERF1/EBP50 tumor suppressor function. Oncogene 26: 5290–5299.
Lazar CS, Cresson CM, Lauffenburger DA, Gill GN . (2004). The Na+/H+ exchanger regulatory factor stabilizes epidermal growth factor receptors at the cell surface. Mol Biol Cell 15: 5470–5480.
Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y et al. (1999). Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99: 323–334.
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI et al. (1997). PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275: 1943–1947.
Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T . (2009). Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene 28: 994–1004.
Maehama T, Dixon JE . (1998). The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378.
Maudsley S, Zamah AM, Rahman N, Blitzer JT, Luttrell LM, Lefkowitz RJ et al. (2000). Platelet-derived growth factor receptor association with Na(+)/H(+) exchanger regulatory factor potentiates receptor activity. Mol Cell Biol 20: 8352–8363.
Molina JR, Hayashi Y, Stephens C, Georgescu MM . (2010a). Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia 12: 453–463.
Molina JR, Morales FC, Hayashi Y, Aldape KD, Georgescu MM . (2010b). Loss of PTEN binding adapter protein NHERF1 from plasma membrane in glioblastoma contributes to PTEN inactivation. Cancer Res 70: 6697–6703.
Morales FC, Takahashi Y, Momin S, Adams H, Chen X, Georgescu MM . (2007). NHERF1/EBP50 head-to-tail intramolecular interaction masks association with PDZ domain ligands. Mol Cell Biol 27: 2527–2537.
Murthy A, Gonzalez-Agosti C, Cordero E, Pinney D, Candia C, Solomon F et al. (1998). NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J Biol Chem 273: 1273–1276.
Pan Y, Weinman EJ, Dai J . (2008). NHERF1 (Na+/H+ exchanger regulatory factor 1) inhibits platelet-derived growth factor signaling in breast cancer cells. Breast Cancer Res 10: R5.
Qiao M, Wang Y, Xu X, Lu J, Dong Y, Tao W et al. (2010). Mst1 is an interacting protein that mediates PHLPPs’ induced apoptosis. Mol Cell 38: 512–523.
Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN . (2009). A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci USA 106: 480–485.
Reczek D, Berryman M, Bretscher A . (1997). Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol 139: 169–179.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM . (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101.
Shenolikar S, Minkoff CM, Steplock DA, Evangelista C, Liu M, Weinman EJ . (2001). N-terminal PDZ domain is required for NHERF dimerization. FEBS Let 489: 233–236.
Shibata T, Chuma M, Kokubu A, Sakamoto M, Hirohashi S . (2003). EBP50, a beta-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology 38: 178–186.
Shimizu K, Okada M, Takano A, Nagai K . (1999). SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett 458: 363–369.
Song J, Bai J, Yang W, Gabrielson EW, Chan DW, Zhang Z . (2007). Expression and clinicopathological significance of oestrogen-responsive ezrin-radixin-moesin-binding phosphoprotein 50 in breast cancer. Histopathology 51: 40–53.
Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH et al. (1997). Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15: 356–362.
Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S et al. (2006). Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 9: 287–300.
Takahashi Y, Morales FC, Kreimann EL, Georgescu MM . (2006). PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J 25: 910–920.
Walker SM, Leslie NR, Perera NM, Batty IH, Downes CP . (2004). The tumour-suppressor function of PTEN requires an N-terminal lipid-binding motif. Biochem J 379: 301–307.
Weinman EJ, Steplock D, Tate K, Hall RA, Spurney RF, Shenolikar S . (1998). Structure-function of recombinant Na/H exchanger regulatory factor (NHE-RF). J Clin Invest 101: 2199–2206.
Acknowledgements
We thank NCI-CA107201 and the corresponding ARRA supplement (M-M. Georgescu), and NCI-CA16672 that partially supported the DNA sequencing and animal breeding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Molina, J., Agarwal, N., Morales, F. et al. PTEN, NHERF1 and PHLPP form a tumor suppressor network that is disabled in glioblastoma. Oncogene 31, 1264–1274 (2012). https://doi.org/10.1038/onc.2011.324
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.324
Keywords
This article is cited by
-
Novel neoplasms associated with syndromic pediatric medulloblastoma: integrated pathway delineation for personalized therapy
Cell Communication and Signaling (2022)
-
Transcription factor SNAI2 exerts pro-tumorigenic effects on glioma stem cells via PHLPP2-mediated Akt pathway
Cell Death & Disease (2022)
-
Novel targetable FGFR2 and FGFR3 alterations in glioblastoma associate with aggressive phenotype and distinct gene expression programs
Acta Neuropathologica Communications (2021)
-
Global activation of oncogenic pathways underlies therapy resistance in diffuse midline glioma
Acta Neuropathologica Communications (2020)
-
A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1
Molecular Cancer (2019)