Abstract
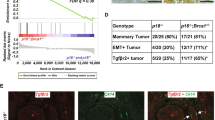
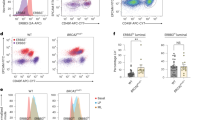
BRCA1 mutation-associated breast cancer originates in oestrogen receptor-alpha-negative (ER−) progenitors in the mammary luminal epithelium. These cells also express high levels of the Kit gene and a recent study demonstrated a correlation between Brca1 loss and Kit over-expression in the mammary epithelium. However, the functional significance of c-Kit expression in the mammary gland is unknown. To address this, c-Kit− and c-Kit+ mammary epithelial subsets were isolated by flow cytometry, characterised for expression of lineage-specific cell markers and functionally analysed by in vitro colony forming and in vivo transplantation assays. The results confirm that the majority of luminal ER− progenitors are c-Kit+, but also that most stem cells and the differentiated cell populations are c-Kit−. A subset of c-Kit+ cells with high proliferative potential was found in the luminal ER+ population, however, suggesting the existence of a distinct luminal ER+ progenitor cell type. Analysis of mouse Brca1 mammary tumours demonstrated that they expressed Kit and its downstream effector Lyn at levels comparable to the most strongly c-Kit+ luminal ER− progenitors. Consistent with c-Kit being a progenitor cell marker, in vitro three-dimensional differentiation of c-Kit+ cells resulted in a loss of c-Kit expression, whereas c-Kit over-expression prevented normal differentiation in vivo. Furthermore, c-Kit was a functional marker of proliferative potential, as c-Kit inhibition by short hairpin knockdown prevented normal epithelial growth and caused cells to undergo apoptosis. Therefore, c-Kit defines distinct progenitor populations in the mammary epithelium and is critical for mammary progenitor survival and proliferation. Importantly, c-Kit is only the second mammary epithelial stem/progenitor marker to be shown to have a functional role in the mammary epithelium and the first marker to be shown to be required for progenitor cell function. The c-Kit signalling network has potential as a target for therapy and/or prevention in BRCA1-associated breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Altiok S, Batt D, Altiok N, Papautsky A, Downward J, Roberts TM et al. (1999). Heregulin induces phosphorylation of BRCA1 through phosphatidylinositol 3-Kinase/AKT in breast cancer cells. J Biol Chem 274: 32274–32278.
Arendt LM, Rugowski DE, Grafwallner-Huseth TA, Garcia-Barchino MJ, Rui H, Schuler LA . (2011). Prolactin-induced mouse mammary carcinomas model estrogen resistant luminal breast cancer. Breast Cancer Res 13: R11.
Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC et al. (2007). Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9: 201–209.
Barton J, Liggett W, Mainwaring M, Hainsworth JD, Simons L, Spigel DR et al. (2006). Phase II pilot trial of imatinib mesylate with weekly docetaxel in metastatic breast cancer. J Clin Oncol 24: 10716.
Baxter LL, Hou L, Loftus SK, Pavan WJ . (2004). Spotlight on spotted mice: a review of white spotting mouse mutants and associated human pigmentation disorders. Pigment Cell Res 17: 215–224.
Brannan CI, Lyman SD, Williams DE, Eisenman J, Anderson DM, Cosman D et al. (1991). Steel-Dickie mutation encodes a c-kit ligand lacking transmembrane and cytoplasmic domains. Proc Natl Acad Sci USA 88: 4671–4674.
Britt KL, Kendrick H, Regan JL, Molyneux G, Magnay FA, Ashworth A et al. (2009). Pregnancy in the mature adult mouse does not alter the proportion of mammary epithelial stem/progenitor cells. Breast Cancer Res 11: R20.
Brizzi MF, Dentelli P, Rosso A, Yarden Y, Pegoraro L . (1999). STAT protein recruitment and activation in c-Kit deletion mutants. J Biol Chem 274: 16965–16972.
Caruana G, Cambareri AC, Ashman LK . (1999). Isoforms of c-KIT differ in activation of signalling pathways and transformation of NIH3T3 fibroblasts. Oncogene 18: 5573–5581.
Chew HK, Barlow W, Albain K, Lew D, Budd T, Allen G et al. (2006). SWOG 0338: A phase II trial of imatinib mesylate in combination with capecitabine in metastatic breast cancer. J Clin Oncol 24: 10529.
Chin H, Arai A, Wakao H, Kamiyama R, Miyasaka N, Miura O . (1998). Lyn physically associates with the erythropoietin receptor and may play a role in activation of the Stat5 pathway. Blood 91: 3734–3745.
Clarke RB, Howell A, Potten CS, Anderson E . (1997). Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57: 4987–4991.
Deberry C, Mou S, Linnekin D . (1997). Stat1 associates with c-kit and is activated in response to stem cell factor. Biochem J 327 (Part 1): 73–80.
Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS . (2003). Stem cells in normal breast development and breast cancer. Cell Prolif 36 (Suppl 1): 59–72.
Driessen RL, Johnston HM, Nilsson SK . (2003). Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp Hematol 31: 1284–1291.
Fernandez-Gonzalez R, Illa-Bochaca I, Welm B, Fleish MC, Werb Z, Ortiz-deSolorzano C et al. (2009). Mapping mammary gland architecture using multi-scale in situ analysis. Integr Biol 1: 80–89.
Gao B, Shen X, Kunos G, Meng Q, Goldberg ID, Rosen EM et al. (2001). Constitutive activation of JAK-STAT3 signaling by BRCA1 in human prostate cancer cells. FEBS Lett 488: 179–184.
Gommerman JL, Rottapel R, Berger SA . (1997). Phosphatidylinositol 3-kinase and Ca2+ influx dependence for ligand-stimulated internalization of the c-Kit receptor. J Biol Chem 272: 30519–30525.
Gommerman JL, Sittaro D, Klebasz NZ, Williams DA, Berger SA . (2000). Differential stimulation of c-Kit mutants by membrane-bound and soluble Steel Factor correlates with leukemic potential. Blood 96: 3734–3742.
Gusterson BA, Ross DT, Heath VJ, Stein T . (2005). Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res 7: 143–148.
Haley BB, Ashfaq R, DeHaas M, Ramaswami A, Sikder K, Tripathy D . (2007). A phase I/II study of imatinib and docetaxel as neoadjuvant therapy in locally advanced breast cancer. J Clin Oncol 25: 11039.
Hayashi S, Kunisada T, Ogawa M, Yamaguchi K, Nishikawa S . (1991). Exon skipping by mutation of an authentic splice site of c-kit gene in W/W mouse. Nucleic Acids Res 19: 1267–1271.
Huang S, Guo YP, May G, Enver T . (2007). Bifurcation dynamics in lineage-commitment in bipotent progenitor cells. Dev Biol 305: 695–713.
Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M et al. (2008). Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics 9: 591.
Kent D, Copley M, Benz C, Dykstra B, Bowie M, Eaves C . (2008). Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin Cancer Res 14: 1926–1930.
Kordon EC, Smith GH . (1998). An entire functional mammary gland may comprise the progeny from a single cell. Development 125: 1921–1930.
Lilla JN, Werb Z . (2010). Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev Biol 337: 124–133.
Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH et al. (2009). Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15: 907–913.
Linnekin D, DeBerry CS, Mou S . (1997). Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J Biol Chem 272: 27450–27455.
Mallepell S, Krust A, Chambon P, Brisken C . (2006). Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA 103: 2196–2201.
Mansson R, Hultquist A, Luc S, Yang L, Anderson K, Kharazi S et al. (2007). Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity 26: 407–419.
Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K . (1995). Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood 85: 641–649.
Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R et al. (2010). BRCA1 Basal-like Breast Cancers Originate from Luminal Epithelial Progenitors and Not from Basal Stem Cells. Cell Stem Cell 7: 403–417.
Molyneux G, Regan J, Smalley MJ . (2007). Mammary stem cells and breast cancer. Cell Mol Life Sci 64: 3248–3260.
Ouchi T, Lee SW, Ouchi M, Aaronson SA, Horvath CM . (2000). Collaboration of signal transducer and activator of transcription 1 (STAT1) and BRCA1 in differential regulation of IFN-gamma target genes. Proc Natl Acad Sci USA 97: 5208–5213.
Pathan NI, Geahlen RL, Harrison ML . (1996). The protein-tyrosine kinase Lck associates with and is phosphorylated by Cdc2. J Biol Chem 271: 27517–27523.
Reith AD, Ellis C, Lyman SD, Anderson DM, Williams DE, Bernstein A et al. (1991). Signal transduction by normal isoforms and W mutant variants of the Kit receptor tyrosine kinase. EMBO J 10: 2451–2459.
Roskoski Jr R . (2005a). Structure and regulation of Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun 338: 1307–1315.
Roskoski Jr R . (2005b). Signaling by Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun 337: 1–13.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML et al. (2006). Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88.
Shivakrupa R, Linnekin D . (2005). Lyn contributes to regulation of multiple Kit-dependent signaling pathways in murine bone marrow mast cells. Cell Signal 17: 103–109.
Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ . (2007). Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol 176: 19–26.
Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ . (2006). CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res 8: R7.
Smalley MJ . (2010). Isolation, culture and analysis of mouse mammary epithelial cells. In: Ward A, Tosh D (eds). Methods in Molecular Biology. Springer: Berlin, pp 139–170.
Smart CE, Wronski A, French JD, Edwards SL, Asselin-Labat ML, Waddell N et al. (2011). Analysis of Brca1-deficient mouse mammary glands reveals reciprocal regulation of Brca1 and c-kit. Oncogene 30: 1597–1607.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D et al. (2006). Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997.
Sun J, Pedersen M, Ronnstrand L . (2008). Gab2 is involved in differential phosphoinositide 3-kinase signaling by two splice forms of c-Kit. J Biol Chem 283: 27444–27451.
Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D et al. (2008). Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol 10: 716–722.
Vafaizadeh V, Klemmt P, Brendel C, Weber K, Doebele C, Britt K et al. (2010). Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation. Stem Cells 28: 928–938.
Vidarsson H, Mikaelsdottir EK, Rafnar T, Bertwistle D, Ashworth A, Eyfjord JE et al. (2002). BRCA1 and BRCA2 bind Stat5a and suppress its transcriptional activity. FEBS Lett 532: 247–252.
Voytyuk O, Lennartsson J, Mogi A, Caruana G, Courtneidge S, Ashman LK et al. (2003). Src family kinases are involved in the differential signaling from two splice forms of c-Kit. J Biol Chem 278: 9159–9166.
Wang H, Shao N, Ding QM, Cui J, Reddy ES, Rao VN . (1997). BRCA1 proteins are transported to the nucleus in the absence of serum and splice variants BRCA1a, BRCA1b are tyrosine phosphoproteins that associate with E2F, cyclins and cyclin dependent kinases. Oncogene 15: 143–157.
Waterhouse DM, Mainwaring M, Barton J, Webb C, Markus TM, Spigel DR et al. (2008). Phase II pilot results of imatinib mesylate with weekly docetaxel in metastatic breast cancer. J Clin Oncol 26: May 20 Suppl; abstr 1090.
Westbury CB, Reis-Filho JS, Dexter T, Mahler-Araujo B, Fenwick K, Iravani M et al. (2009). Genome-wide transcriptomic profiling of microdissected human breast tissue reveals differential expression of KIT (c-Kit, CD117) and oestrogen receptor-alpha (ERalpha) in response to therapeutic radiation. J Pathol 219: 131–140.
Young SM, Cambareri AC, Odell A, Geary SM, Ashman LK . (2007). Early myeloid cells expressing c-KIT isoforms differ in signal transduction, survival and chemotactic responses to Stem Cell Factor. Cell Signal 19: 2572–2581.
Yu M, Luo J, Yang W, Wang Y, Mizuki M, Kanakura Y et al. (2006). The scaffolding adapter Gab2, via Shp-2, regulates kit-evoked mast cell proliferation by activating the Rac/JNK pathway. J Biol Chem 281: 28615–28626.
Acknowledgements
We thank Eric So for the c-Kit cDNA, Fredrik Wallberg for help with flow cytometry analysis and Jorge Reis-Filho and Arno Gauthier for assistance with assessment of Lyn staining on mouse tumours. This work was funded by Breakthrough Breast Cancer. We acknowledge NHS funding to the NIHR Biomedical Research Centre. This work was funded by Breakthrough Breast Cancer. We acknowledge NHS funding to the NIHR Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Regan, J., Kendrick, H., Magnay, FA. et al. c-Kit is required for growth and survival of the cells of origin of Brca1-mutation-associated breast cancer. Oncogene 31, 869–883 (2012). https://doi.org/10.1038/onc.2011.289
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.289
Keywords
This article is cited by
-
Loss of SNAI1 induces cellular plasticity in invasive triple-negative breast cancer cells
Cell Death & Disease (2022)
-
Induction of apoptosis in SGC-7901 cells by iridium(III) complexes via endoplasmic reticulum stress-mitochondrial dysfunction pathway
JBIC Journal of Biological Inorganic Chemistry (2022)
-
New C3H KitN824K/WT cancer mouse model develops late-onset malignant mammary tumors with high penetrance
Scientific Reports (2022)
-
Single cell transcriptome atlas of mouse mammary epithelial cells across development
Breast Cancer Research (2021)
-
Estrogen receptor-α signaling in post-natal mammary development and breast cancers
Cellular and Molecular Life Sciences (2021)