Abstract
The serine/threonine kinase Akt is frequently activated in human cancers and is considered an attractive therapeutic target. However, the relative contributions of the different Akt isoforms to tumorigenesis, and the effect of their deficiencies on cancer development are not well understood. We had previously shown that Akt1 deficiency is sufficient to markedly reduce the incidence of tumors in Pten+/− mice. Particularly, Akt1 deficiency inhibits endometrial carcinoma and prostate neoplasia in Pten+/− mice. Here, we analyzed the effect of Akt2 deficiency on the incidence of tumors in Pten+/− mice. Relative to Akt1, Akt2 deficiency had little-to-no effect on the incidence of prostate neoplasia, endometrial carcinoma, intestinal polyps and adrenal lesions in Pten+/− mice. However, Akt2 deficiency significantly decreased the incidence of thyroid tumors in Pten+/−, which correlates with the relatively high level of Akt2 expression in the thyroid. Thus, unlike Akt1 deletion, Akt2 deletion is not sufficient to markedly inhibit tumorigenesis in Pten+/− mice in most tested tissues. The relatively small effect of Akt2 deletion on the inhibition of tumorigenesis in Pten+/− mice could be explained, in part, by an insufficient decrease in total Akt activity, due to the relatively lower Akt2 versus Akt1 expression, and relatively high blood insulin levels in Pten+/−Akt2−/− mice. The relatively high blood insulin levels in Pten+/−Akt2−/− mice may elevate the activity of Akt1, and possibly Akt3, thus, limiting the reduction of total Akt activity and preventing this activity from dropping to a threshold level required to inhibit tumorigenesis.
Similar content being viewed by others
Introduction
The tumor suppressor phosphatase and tensin homolog (PTEN) is a phospholipid phosphatase that negates the activity of phosphatidylinositol 3-kinase by de-phosphorylating the D3 position of the inositol ring of phosphoinositides phosphorylated by phosphatidylinositol 3-kinase. Inactivation of PTEN occurs frequently in human cancers, particularly in prostate carcinoma, endometrial carcinoma and glioblastoma (Cairns et al., 1997; Cantley and Neel, 1999; Muller et al., 2000; Mutter et al., 2000; Parsons and Simpson, 2003). Pten+/− mice recapitulate PTEN deficiency in human cancer as manifested by the high incidence of high-grade prostate intraepithelial neoplasia (PIN), and endometrial carcinoma in these mice (Di Cristofano et al., 1998; Suzuki et al., 1998; Podsypanina et al., 1999; Stambolic et al., 2000; Chen et al., 2006). In addition, Pten+/− mice develop a high incidence of adrenal medullary neoplasia, thyroid neoplasia, intestinal polyps and lymphoid hyperplasia (Chen et al., 2006).
A major downstream effector of PTEN is Akt, which is hyperactivated by PTEN deficiency. Mammalian cells have three genes that encode three Akt isoforms (Akt1–3). The three Akt isoforms have a high degree of amino acid sequence identity, and appear to have similar kinase specificity in in vitro kinase assays (Walker et al., 1998). The individual Akt isoform knockout mice display different phenotypes, which could be explained by the relative expression of the three Akt isoforms in the affected tissues and organs (reviewed in (Bhaskar and Hay, 2007)). However, isoform-specific function of the different Akt isoforms cannot be completely excluded. Indeed, it was reported that Akt1 and Akt2 might be functionally different with respect to cell invasion, migration and metastasis (Bhaskar and Hay, 2007). It was also reported recently that different Akt isoforms regulate distinct subsets of microRNAs (Iliopoulos et al., 2009), and that the actin-bundling protein, palladin, is a specific substrate of Akt1 and not of Akt2 (Chin and Toker, 2010). The deletion of Akt1 in mice does not elicit any major severe physiological consequences (Chen et al., 2001; Cho et al., 2001b). The deletion of Akt2 elicits insulin resistance with a significant elevation of insulin levels (Cho et al., 2001a; Garofalo et al., 2003; Chen et al., 2009), but the haploinsufficiency Akt1 together with Akt2 deficiency elicits a severe diabetic phenotype (Chen et al., 2009). Because Akt is an attractive target for cancer therapy, and Akt-isoform-specific inhibitors are being developed (Li et al., 2009; Lindsley, 2010), it is important to know the effect of Akt-isoform-specific inhibition on tumor development.
Previously, we had shown that Akt1 deficiency is sufficient to inhibit all the neoplastic lesions developed in Pten+/− mice. Even haplodeficiency of Akt1 was sufficient to attenuate significantly the development of high-grade PIN and endometrial carcinoma in Pten+/− mice (Chen et al., 2006). Akt1 deficiency is also sufficient to inhibit skin carcinogensis, and salivary and mammary gland tumors developed in MMTV-Ras and MMTV-neu mice (Skeen et al., 2006; Ju et al., 2007). However, unlike Akt1 deletion, the deletion of Akt2 does not appear to inhibit mammary gland tumorigenesis, and even accelerates it (Maroulakou et al., 2007). Here, we examined the effect of Akt2 deficiency on the development of tumors in Pten+/− mice. We found that in most tested tissues, Akt2 deletion did not inhibit tumorigenesis. Although Akt2 is expressed to a lesser extent than Akt1 in these tissues, this reduced expression cannot fully explain why the deletion of Akt2 was not sufficient to attenuate the development of tumors inhibited by Akt1 deletion in Pten+/− mice. We propose that the relatively high level of blood insulin in Pten+/−Akt2−/− mice prohibits the reduction of total Akt activity to a threshold level required to inhibit tumorigenesis by elevating the activity of other Akt isoforms.
Results
Akt2 deficiency does not inhibit the development of high-grade PIN in PTEN heterozygous mice
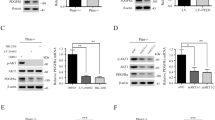
Pten+/− male mice have high incidence of high-grade PIN when they reach 1 year of age (Chen et al., 2006). We determined the development of PIN in the three mouse prostate lobes of 12-month-old Pten+/–, Pten+/–Akt2–/– mice using histopathological criteria and bromodeoxyuridine (BrdU) incorporation as previously described (Chen et al., 2006). High-grade lesions (PIN3 and PIN4) were monitored as previously described (Chen et al., 2006). In the anterior lobe of Pten+/− mice, we observed 100% incidence of PIN4 lesions, which was not affected by Akt2−/− deletion (Figure 1a). In the dorsolateral lobe we observed about 25% incidence of PIN3 lesions and about 75% incidence of PIN4 lesions. In the dorsolateral lobe of Pten+/−Akt2−/− mice, there was about 30% incidence of PIN3 and 70% incidence of PIN4 lesions. The incidence of PIN4 lesions in the ventral lobe was close to 100% and was only slightly decreased by Akt2−/− deletion, which also slightly increased the incidence of PIN3 lesions. Overall, Akt2 deletion does not appear to markedly affect the development and incidence of high-grades PIN in the prostate of Pten+/− mice. These results were corroborated by the examination of BrdU incorporation that showed no significant difference between Pten+/− and Pten+/−Akt2−/− prostates (Figure 1b). In normal prostate, keratin 14 (K14) can only be detected in basal cells and is absent in the luminal epithelium. It was shown, however, that high-grade PIN in Pten+/− mice are characterized by increased expression of K14 in the lumen (Park et al., 2002). In our previous analysis we established intense K14 staining as independent criterion for high-grade PIN in the mouse prostate. In the normal prostate, K14-positive cells were confined to the basal layer, whereas in high-grade PIN, there were many large K14-positive cells in the lumen of the duct (Chen et al., 2006). We therefore monitored the staining for K14 and found that it corroborated the histopathological analyses and the BrdU incorporation (Figure 1c).
Akt2 deficiency does not impair the development of high-grade PIN in Pten+/– mice. (a) Incidence of PIN3 and PIN4 lesions in the three prostate lobes (A, anterior, DL, dorsolateral and V, ventral) of Pten+/– and Pten+/–Akt2–/– mice. The numbers of mice in each group are indicated. (b) BrdU incorporation in the prostate lobes of Pten+/– and Pten+/–Akt2–/– mice. The numbers of mice in each group are indicated in parentheses. BrdU-positive cells were counted as described in Materials and methods. P-values were calculated for each prostate lobe in each genotype. (c) Quantification of K14 staining, from five mice of each genotype as described in Materials and methods.
In contrast to the Akt2 deletion, we had shown previously that Akt1 deletion decreased the incidence of PIN4 lesions in the anterior and dorsolateral lobes of the prostate in Pten+/− mice by a 7- to 8-fold factor, and completely diminished the incidence of high-grade PIN in the ventral lobe. Even the haplodeficiency of Akt1 decreased the incidence of PIN4 lesions in the prostate of Pten+/− mice by a factor of 2 to 4 (Chen et al., 2006).
Akt2 deficiency is not sufficient to inhibit the development of endometrial carcinoma in Pten+/– mice
PTEN dysfunction is one of the most frequently occurring genetic lesions in endometrial carcinoma (Tashiro et al., 1997). Female Pten+/– mice have a high incidence of endometrial neoplasia by 9 months of age. The development of severe lymphoid hyperplasia in Pten+/– female mice ultimately leads to their death before 1 year of age (Di Cristofano et al., 1998, 2001; Podsypanina et al., 1999; Stambolic et al., 2000), precluded analysis of older female mice.
Previously (Chen et al., 2006), we classified endometrial epithelial lesions in the endometrium of Pten+/− mice as simple hyperplasia, mild atypical hyperplasia, moderate atypical hyperplasia, complex atypical hyperplasia and focal carcinoma in situ (CIS) ((Chen et al., 2006), Figure 2a). In our current histopathological analyses of 9-month-old female mice, we found that out of 43 Pten+/− mice, 24 (about 56%) display the highest grade as CIS, and 10 (about 23%) the highest grade as AH3. Out of 36 Pten+/−Akt2−/− mice, 18 (50%) display CIS and 9 (about 25%) display AH3 (Figure 2b). Though the incidence of CIS is slightly lower in Pten+/−Akt2−/− mice, overall, Akt2 deletion does not appear to inhibit the development of the neoplastic lesions in the endometrium of Pten+/− mice. The histopathological results were corroborated by the analysis of BrdU incorporation (Figure 2c). In contrast to these results, our previous analyses showed that the deletion of Akt1 reduced the incidence of endometrial CIS in Pten+/− mice by almost fourfold. Even haplodeficiency of Akt1 was sufficient to reduce the incidence of endometrial CIS more than twofold (Chen et al., 2006).
Akt2 deficiency is insufficient to inhibit the development of endometrial carcinoma in Pten+/– mice. (a) Histological sections representing the different grades of endometrial neopplasia in Pten+−/− mice (AH, atypical hyperplasia, CIS, carcinoma in situ). The source of the tissue sections is indicated. (b) Incidence of endometrial neoplasia in Pten+/–, Pten+/–Akt2–/– and wild-type mice. Total number of mice examined in each group is indicated in parentheses. (c) BrdU incorporation in the uteri of Pten+/–, Pten+/–Akt2–/– and wild-type mice. The numbers of mice in each group are indicated in parentheses. P-values are indicated.
The effect of Akt2 deficiency on the development of adrenal medullary neoplasia and intestinal polyps in Pten+/– mice
Both female and male Pten+/− mice develop bilateral adrenal medullary tumors (Chen et al., 2006). In our previous studies we found that the size of the adrenal medulla, which was directly correlated with the size and grade of the tumors in Pten+/– mice, was significantly decreased in Pten+/–Akt1–/– mice (Chen et al., 2006). We therefore quantified the diameter of the adrenal medulla as an indirect measure of tumor size. In both female and male mice, the deficiency of Akt2 slightly decreased the diameter of the adrenal medulla in Pten+/− mice. However, unlike the deletion of Akt1 that significantly reduced BrdU incorporation in the adrenal glands of Pten+/− mice (Chen et al., 2006), the reduction of BrdU incorporation by Akt2 deletion is not significant, and no significant change in mitotic index was observed (Figure 3b, and data not shown).
Effect of Akt2 deficiency on tumor development in the adrenal gland and on the number of polyps in the small intestine of Pten+/– mice. (a) The diameter of the adrenal glands in 9-month-old female or 12-month-old male, Pten+/–, Pten+/–Akt2–/– and wild-type mice. The numbers of mice are indicated in parentheses. (b) Quantification of BrdU incorporation in the adrenal medulla of Pten+/– and Pten+/–Akt2–/– mice. BrdU analysis was carried out as described in Figure 1b. (c) The deficiency of Akt2 did not reduce the number of polyps in the small intestine of Pten+/– mice. Quantification of the number of intestinal polyps in 9-month-old female and 12-month-old male mice. The number of polyps±s.d. per mouse is shown. The numbers of mice are indicated in parentheses (F, female; M, male).
As was previously shown, Pten+/− mice have a high incidence of intestinal polyps. These polyps seemed to be adenomatous polyps (tubular adenoma) or hamartomatous polyps, and their incidence was significantly reduced by the deletion of and even by the haplodeficiency of Akt1 in Pten+/− mice (Chen et al., 2006). In our current analyses, we found that 12-month-old male and 9-month-old female Pten+/− mice develop intestinal polyps with ∼80% and 90% incidence, respectively, similar to previous observations (Chen et al., 2006). This incidence, however, was not significantly decreased by the deletion of Akt2. Quantification of the average number of polyps per mouse did not show a statistically significant decrease in Pten+/−Akt2−/− mice (Figure 3c). In contrast, both haplodeficiency and a complete deletion of Akt1 in Pten+/− mice reduced the average number of polyps per mouse substantially (Chen et al., 2006).
Akt2 deficiency inhibits the development of thyroid tumors in Pten+/− mice
Previous studies showed a high incidence of thyroid tumors in Pten+/− mice (Podsypanina et al., 1999; Chen et al., 2006). Two major neoplastic lesions were defined. Transformed glands that tend to blend into the surrounding normal-looking thyroid follicles were defined as separated transformed glands. Aggregated transformed glands with nodule formation that, based on their morphology, are consistent with thyroid adenomatoid nodules or multiple adenomas were defined as tumors (Chen et al., 2006). Previous examination of 11-month-old Pten+/− male mice found that 80% of the mice developed thyroid tumors, and 20% of the mice developed separated transformed glands, which is the lower grade lesion. The deletion of Akt1 reduced by almost twofold the incidence of thyroid tumors in Pten+/− mice (Chen et al., 2006). In our current studies we found consistently that 75% of 12-month-old Pten+/− male mice developed thyroid tumors and 25% developed only separated transformed glands (Figure 4). The deletion of Akt2 in Pten+/− male mice reduced the percentage of mice with tumors to 42%, but the percentage of mice with separated transformed glands was increased to 40%, and 18% of Pten+/−Akt2−/− mice were free of any neoplastic lesion (Figure 4). In 9-month-old female Pten+/−, the deletion of Akt2 decreases the percentage of mice with tumors from about 53% to about 42% (Figure 4). These results are very similar to those obtained with Akt1 deletion (Chen et al., 2006), indicating that the deletion of either Akt1 or Akt2 reduced thyroid tumorigenicity in Pten+/− mice to the same extent.
The deletion of Akt2 in Pten+/− mice did not significantly reduce total Akt activity, except in the thyroid
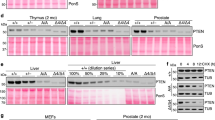
To understand why Akt2 deletion has only minimal effect on tumorigenesis in Pten+/− mice, we examined the effect of Akt2 deletion on total Akt activity. Surprisingly we did not find any significant reduction in total Akt activity after the deletion of Akt2 in Pten+/− prostate, uterus and adrenal glands (Figures 5a, b and d). We previously showed strong pAkt immunostaining on the plasma membrane of cells in histological sections of high-grade neoplastic lesions in the prostate of Pten+/− mice (Chen et al., 2006). However, strong plasma membrane staining of pAkt was not diminished in histological sections derived from Pten+/−Akt2−/− prostate (Figure 5a, left panel). In contrast to the prostate, uterus and adrenal, we observed a decrease of about 50% in total Akt activity in the thyroid (Figures 5c and d). Thus, the effect of Akt2 deletion on tumorigenesis in Pten+/− mice could be directly correlated to its effect on the reduction of total Akt activity.
The deletion of Akt2 in Pten+/− mice did not significantly reduce total Akt activity except in the thyroid. Six- to nine-month-old female and male mice were killed and pAkt was assessed by immunoblotting of proteins extracted from various tissues. (a) Left panel —representative immunoblot showing the level of Akt phosphorylated at Ser473, in prostate tissues isolated from Pten+/− and Pten+/−Akt2−/− mice. The immunoblot was also subjected to anti-Akt2 antibodies and anti-pan Akt antibodies. Right panel—immunohistochemistry, using anti-pS473-Akt, of histological sections from the anterior and dorsolateral prostate lobes of Pten+/− and Pten+/−Akt2−/− mice. (b) Representative immunoblot showing the level of Akt phosphorylated at Ser473 in uteri isolated from Pten+/− and Pten+/−Akt2−/− mice. The immunoblot was also subjected to anti-Akt2 antibodies and anti-pan Akt antibodies. (c) Representative immunoblot showing the level of Akt phosphorylated at Ser473 in thyroids isolated from Pten+/− and Pten+/−Akt2−/− mice. The immunoblot was also subjected to anti-Akt2 antibodies and anti-pan Akt antibodies. (d) Quantification of pAkt/tubulin ratio in prostate samples from three Pten+/− and Pten+/−Akt2−/− mice, in uteri from six Pten+/− and four Pten+/−Akt2−/− mice, in the adrenal glands from six Pten+/− and five Pten+/−Akt2−/− mice, and in the thyroids from five Pten+/− and Pten+/−Akt2−/− mice. (e) Representative immunoblot showing phosphorylation of PRAS40 at threonine 246 by Akt. G6PD was used as a loading control. (f) Quantification of Akt1 versus Akt2 mRNA expression using q-PCR (Chen et al., 2006).
Previously, we analyzed by quantitative RT–PCR the relative expression of Akt1 and Akt2 (Chen et al., 2006), shown in Figure 5e, as a Akt1/Akt2 ratio. On the basis of this ratio, deletion of Akt2 can be expected to reduce total Akt activity by about 30% in the uterus, by close to 40% in the prostate, by close to 50% in the adrenal glands and by about 70% in the thyroid. Thus, total Akt activity was not decreased to the level expected from the relative expression of Akt2. However, when the immunoblot was subjected to pan-Akt antibodies, we did not observe the expected decrease in total Akt in all tissues in which Akt2 was deleted (Figures 5a–c, and Supplementary Figure 1). As we did not observe any elevation in Akt1 protein expression in these tissues (Supplementary Figure 1), we suspect that the pan-Akt antibody has a preference for Akt1, and therefore cannot be used to accurately measure the reduction in total Akt proteins.
To further corroborate the effect of Akt2 deficiency on total Akt activity, we used the phosphorylation of proline-rich protein PRAS40 by Akt as readout for total Akt activity. Consistently, we found that there was no significant reduction in the phosphorylation of PRAS40 in Pten+/−Akt2−/− tissues with relative to Pten+/− tissues, except in the thyroid (Figure 5e).
Akt2−/− mice have over a 10-fold higher level of insulin than wild-type level because they display insulin resistance (Chen et al., 2009). In Pten+/−Akt2−/− mice, insulin level is reduced compared with Akt2−/− mice (Chen et al., 2009), but when compared with Pten+/− mice, insulin level is elevated about sixfold in male mice and a little more than threefold in female mice (Figure 6a). Therefore, the discrepancy between the expected reduction in total Akt activity after Akt2 deletion in Pten+/− mice could also be explained by the relatively high blood insulin level in Pten+/−Akt2−/− mice, which elevates the activity of the other two Akt isoforms. To confirm this assumption we measured tyrosine 608 phosphorylation of insulin receptor substrate-1, as readout for insulin receptor activity. The phosphorylated tyrosine 608 binds and activates phosphatidylinositol 3-kinase. We found that indeed in Pten+/−Akt2−/− tissues pTyr608 is markedly elevated when compared with Pten+/− tissues (Figure 6b). We therefore concluded that the relatively lower expression of Akt2 versus Akt1 in combination with the high level of insulin signaling in Pten+/−Akt2−/− mice prohibited a marked reduction in tumorigenesis in these mice.
Akt2-deficiency in Pten+/− mice elevates blood insulin levels and phosphorylation insulin receptor substrate-1 on tyrosine 608. (a) Serum insulin level was measured in 6-month-old WT, Pten+/− (P+/−), and Pten+/−Akt2−/− mice as described in Materials and methods. Number of mice is indicated. Bars represent the s.e.m. (b) Representative immunoblot showing the phosphorylation of insulin receptor substrate-1 on tyrosine 608. G6PD was used as a loading control.
Discussion
Previous results showed that Akt1 deletion alone was sufficient to significantly attenuate tumor development in Pten+/− mice, and in many cases even the haplodeficiency of Akt1 significantly attenuated the development of tumors in Pten+/− mice (Chen et al., 2006). By contrast, the results described above showed that Akt2 deficiency is not sufficient to inhibit significantly the development of tumors in Pten+/−, except in the thyroid. One possibility is that the relative expression of Akt1 versus Akt2 in the different tissues determines their differential effect on tumorigenesis. In tissues such as prostate, uterus and intestine, in which the predominantly expressed Akt isoform is Akt1, Akt2 deletion does not appear to significantly or markedly affect tumor development in Pten+/− mice. In these tissues, Akt1 deletion markedly and significantly attenuated tumor development. In the thyroid in which Akt2 deletion markedly attenuated tumor development, Akt2 is the predominantly expressed Akt isoform. Thus, at a glance, the differential effect of the deletion of the two Akt isoforms on tumorigenesis in Pten+/− mice is correlated with their relative level of expression. However, as indicated above, even the haplodeficiency of Akt1 was sufficient to markedly attenuate the development of neoplasia in the prostate, endometrium and intestine. Thus, on the basis of the relative expression of Akt1 and Akt2 in these tissues, Akt2 deletion is expected to reduce total Akt activity to the same extent as the haplodeficiency of Akt1. Consequently Akt2 deletion is expected to have a similar effect on tumor development to that observed by the Akt1 haplodeficiency. Furthermore, although Akt2 is the major Akt isoform expressed in the thyroid, Akt2 deficiency reduced tumor development in the thyroid by the same extent achieved by Akt1 deficiency (Chen et al., 2006).
The inability of Akt2 deficiency to efficiently inhibit tumor development, as should be expected, based on its relative expression, could be due to the relatively high level of blood insulin in Pten+/−Akt2−/− mice. The high levels of insulin in Akt2−/− mice prohibited the reduction in total Akt activity as it elevated the activity of the two remaining Akt isoforms. Total Akt activity was markedly reduced by Akt2 deletion in Pten+/− thyroid consistent with twofold higher level of Akt2 versus Akt1 in the thyroid. However, on the basis of the relative level of expression it is expected that total Akt activity in the Pten+/−Akt2−/− thyroid should be reduced by about two-third. Supporting this possibility are the findings of an association of hyperinsulinemia with increased incidence of neoplasia in several animal models of cancer (Gallagher and LeRoith, 2010; Novosyadlyy et al., 2010). The hyperinsulinemia in Akt2−/− mice may also explain the observed acceleration of mammary gland tumor development by Akt2 deletion in mouse models for breast cancer (Maroulakou et al., 2007).
Cell autonomous functional differences between Akt1 and Akt2 that could account for the differences between the effects of Akt1 and Akt2 deletion on tumor development in Pten+/− cannot be completely excluded. Indeed, functional differences between Akt1 and Akt2 were reported (Irie et al., 2005; Iliopoulos et al., 2009). Further studies using cell type-specific conditional knockouts of Akt2 are required to determine whether the inability of Akt2 deletion to inhibit tumor development is cell autonomous.
The results presented here have implications for cancer therapy. The results suggest that the ablation of Akt2 will not be beneficial for cancer therapy in general but could be beneficial for certain types of cancers in which Akt2 is the major Akt-expressed isoform, such as thyroid cancer in which it was reported to have a particular role in pathogenesis and progression (Ringel et al., 2001). Inhibitors of Akt that markedly inhibit Akt2 may not be useful for prostate and endometrial cancers that have dysfunctional PTEN, unless they also markedly inhibit Akt1. Ideally, selective inhibitors of Akt1 should be used for the treatment of prostate and endometrial cancers that display dysfunctional PTEN. The recent development of small-molecule inhibitors that preferentially inhibit Akt1 (Li et al., 2009; Lindsley, 2010) could address this issue.
Materials and methods
Mice
Pten+/− mice and Akt2−/− mice used in these studies are in C57BL6 background and were previously described (Chen et al., 2006, 2009).
Histology and immunohistochemistry
The prostate or uterus, adrenal gland, small and large intestine and thyroid were harvested and tissues were fixed by immersion in 10% buffered formalin, then processed and embedded in paraffin sections (5 μm) and subjected to hematoxylin and eosin, immunoperoxidase or immunofluorescence staining. Sections were examined by light microscopy or Zeiss LSM 510 Meta Confocal microscopy.
Immunohistochemistry was conducted as previously described (Chen et al., 2006). The following primary antibodies were used: rabbit anti-pS473-Akt, (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-PTEN (Biosource, Camarillo, CA, USA), mouse anti-BrdU (Dako, Carpinteria, CA, USA) and rabbit anti-cytokeratin 14 (Novocastra Laboratory, Newcastle upon Tyne, UK). For pAkt and PTEN staining, the related blocking peptide was mixed with the primary antibody as extra-negative control to verify the specificity of these antibodies. Prostate paraffin sections obtained from different genotypes of mice were stained with anti-K14. The result of staining were scored as follows:
-
0.0: no detectable positive cell in whole section.
-
0.5: Few lightly stained slim satellite positive cells.
-
1.0: Many separated triangles shaped strong positive cells.
-
2.0: Positive cells attached to each other and form a line at the base of gland.
-
3.0: The positive cells are not limited to the base of gland, and some positive cells are also present inside the gland in the lumen.
-
4.0: Strong positive cells composed of variable size of sheets.
BrdU incorporation assay
Mice were given intraperitoneal injections of 0.5 mg BrdU per 10 g body weight for 1 h before killing. After dewax and rehydration, the paraffin sections were digested by pepsin followed by digestion with EcoR1 and Exonuclease III. Slides were then incubated with anti-BrdU and processed for immunohistochemistry. BrdU-labeled cells were counted from five fields at × 250 magnification for prostate and uterus and × 400 magnification for adrenal medulla. All counts were taken from the highest labeled area of all sections.
Measurement of the size of adrenal and adrenal medulla
The longest diameter of adrenal was recorded and the images of adrenal were taken by using confocal microscopy according to the different intensity of autofluorescence from eosin to distinguish the cortex and medulla area. An arbitrary number of the size of medullary areas was obtained by using Image J software.
Immunoblotting
For immunoblotting, tissues were grounded and lysed in 200 μl of lysis buffer (20 mM Tris–HCl, pH 7.5), 100 mM KCl, 20 mM glycerol phosphate, 1 mM dithiothreitol, 0.25 mM Na3VO4, 10 mM NaF, 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium pyrophosphate, 10 nM okadaic acid and proteinase inhibitor cocktail (Complete mini from Roche Applied Science, Indianapolis, IN, USA) was added. Insoluble materials were removed by centrifugation. Samples were subjected to immunoblotting using anti-pS473-Akt, anti-pan-Akt, anti-Akt1, anti-Akt2 and anti-pT246-PRAS40 antibodies from Cell Signaling, and anti-pTyr608-insulin receptor substrate-1 antibody from Millipore (Billerica, MA, USA).
Measurement of insulin levels
Serum insulin levels were measured as described previously (Chen et al., 2009), using enzyme-linked immunosorbent assay (Millipore, Cat.# EZRMI-13K) according to the manufacturer's instructions.
Data analysis
Data were entered into Microsoft EXCEL spreadsheet. P-values were calculated with Student's t-test. Error bars represent s.d.
References
Bhaskar PT, Hay N . (2007). The two TORCs and Akt. Dev Cell 12: 487–502.
Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG et al. (1997). Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 57: 4997–5000.
Cantley LC, Neel BG . (1999). New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 96: 4240–4245.
Chen ML, Xu PZ, Peng X, Chen WS, Guzman G, Yang X et al. (2006). The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev 20: 1569–1574.
Chen WS, Peng XD, Wang Y, Xu PZ, Chen ML, Luo Y et al. (2009). Leptin deficiency and beta-cell dysfunction underlie type 2 diabetes in compound Akt knockout mice. Mol Cell Biol 29: 3151–3162.
Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T et al. (2001). Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev 15: 2203–2208.
Chin YR, Toker A . (2010). The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell 38: 333–344.
Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw III EB et al. (2001a). Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731.
Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ . (2001b). Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276: 38349–38352.
Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP . (2001). Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet 27: 222–224.
Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP . (1998). Pten is essential for embryonic development and tumour suppression. Nat Genet 19: 348–355.
Gallagher EJ, LeRoith D . (2010). Insulin, insulin resistance, obesity, and cancer. Curr Diab Rep 10: 93–100.
Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL et al. (2003). Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 112: 197–208.
Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K et al. (2009). MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal 2: ra62.
Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N et al. (2005). Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol 171: 1023–1034.
Ju X, Katiyar S, Wang C, Liu M, Jiao X, Li S et al. (2007). Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci USA 104: 7438–7443.
Li Y, Liang J, Siu T, Hu E, Rossi MA, Barnett SF et al. (2009). Allosteric inhibitors of Akt1 and Akt2: discovery of [1,2,4]triazolo[3,4-f][1,6]naphthyridines with potent and balanced activity. Bioorg Med Chem Lett 19: 834–836.
Lindsley CW . (2010). The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation: a 2009 update. Curr Top Med Chem 10: 458–477.
Maroulakou IG, Oemler W, Naber SP, Tsichlis PN . (2007). Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res 67: 167–177.
Muller M, Rink K, Krause H, Miller K . (2000). PTEN/MMAC1 mutations in prostate cancer. Prostate Cancer Prostatic Dis 3: S32.
Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA et al. (2000). Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 92: 924–930.
Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y et al. (2010). Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res 70: 741–751.
Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, Shen MM et al. (2002). Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol 161: 727–735.
Parsons R, Simpson L . (2003). PTEN and cancer. Methods Mol Biol 222: 147–166.
Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM et al. (1999). Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA 96: 1563–1568.
Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H et al. (2001). Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res 61: 6105–6111.
Skeen JE, Bhaskar PT, Chen CC, Chen WS, Peng XD, Nogueira V et al. (2006). Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell 10: 269–280.
Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW . (2000). High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res 60: 3605–3611.
Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I et al. (1998). High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol 8: 1169–1178.
Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI et al. (1997). Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res 57: 3935–3940.
Walker KS, Deak M, Paterson A, Hudson K, Cohen P, Alessi DR . (1998). Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochem J 331: 299–308.
Acknowledgements
These studies were supported by NIH grants, CA090764, AG016927 and AG025953 to NH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Xu, PZ., Chen, ML., Jeon, SM. et al. The effect Akt2 deletion on tumor development in Pten+/− mice. Oncogene 31, 518–526 (2012). https://doi.org/10.1038/onc.2011.243
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.243
Keywords
This article is cited by
-
Differential effects of single fatty acids and fatty acid mixtures on the phosphoinositide 3-kinase/Akt/eNOS pathway in endothelial cells
European Journal of Nutrition (2022)
-
Akt isoform-specific effects on thyroid cancer development and progression in a murine thyroid cancer model
Scientific Reports (2020)
-
Akt as a target for cancer therapy: more is not always better (lessons from studies in mice)
British Journal of Cancer (2017)
-
PI3K/AKT Pathway and Its Mediators in Thyroid Carcinomas
Molecular Diagnosis & Therapy (2016)
-
Molecular pathogenesis and mechanisms of thyroid cancer
Nature Reviews Cancer (2013)