Abstract
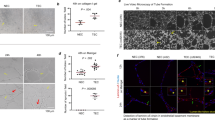
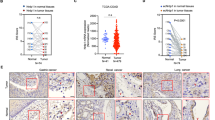
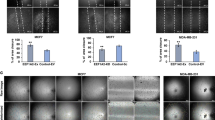
Changes in intracellular calcium [Ca2+]i levels control critical cytosolic and nuclear events that are involved in the initiation and progression of tumor angiogenesis in endothelial cells (ECs). Therefore, the mechanism(s) involved in agonist-induced Ca2+i signaling is a potentially important molecular target for controlling angiogenesis and tumor growth. Several studies have shown that blood vessels in tumors differ from normal vessels in their morphology, blood flow and permeability. We had previously reported a key role for arachidonic acid (AA)-mediated Ca2+ entry in the initial stages of tumor angiogenesis in vitro. In this study we assessed the mechanism involved in AA-induced EC migration. We report that TRPV4, an AA-activated channel, is differentially expressed in EC derived from human breast carcinomas (BTEC) as compared with ‘normal’ EC (HMVEC). BTEC display a significant increase in TRPV4 expression, which was correlated with greater Ca2+ entry, induced by AA or 4αPDD (a selective TRPV4 agonist) in the tumor-derived ECs. Wound-healing assays revealed a key role of TRPV4 in regulating cell migration of BTEC but not HMVEC. Knockdown of TRPV4 expression completely abolished AA-induced BTEC migration, suggesting that TRPV4 mediates the pro-angiogenic effects promoted by AA. Furthermore, pre-incubation of BTEC with AA induced actin remodeling and a subsequent increase in the surface expression of TRPV4. This was consistent with the increased plasma membrane localization of TRPV4 and higher AA-stimulated Ca2+ entry in the migrating cells. Together, the data presented herein demonstrate that: (1) TRPV4 is differentially expressed in tumor-derived versus ‘normal’ EC; (2) TRPV4 has a critical role in the migration of tumor-derived but not ‘normal’ EC migration; and (3) AA induces actin remodeling in BTEC, resulting in a corresponding increase of TRPV4 expression in the plasma membrane. We suggest that the latter is critical for migration of EC and thus in promoting angiogenesis and tumor growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Antoniotti S, Fiorio Pla A, Pregnolato S, Mottola A, Lovisolo D, Munaron L . (2003). Control of endothelial cell proliferation by calcium influx and arachidonic acid metabolism: a pharmacological approach. J Cell Physiol 197: 370–378.
Becker D, Bereiter-Hahn J, Jendrach M . (2009). Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur J Cell Biol 88: 141–152.
Becker D, Blase C, Bereiter-Hahn J, Jendrach M . (2005). TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci 118: 2435–2440.
Bhati R, Patterson C, Livasy CA, Fan C, Ketelsen D, Hu Z et al. (2008). Molecular characterization of human breast tumor vascular cells. Am J Pathol 172: 1381–1390.
Birnbaumer L . (2009). The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol 49: 395–426.
Boels K, Glassmeier G, Herrmann D, Riedel IB, Hampe W, Kojima I et al. (2001). The neuropeptide head activator induces activation and translocation of the growth-factor-regulated Ca(2+)-permeable channel GRC. J Cell Sci 114: 3599–3606.
Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G . (2003). Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J 17: 1159–1161.
Bussolati B, Grange C, Camussi G . (2011). Tumor exploits alternative strategies to achieve vascularization. Faseb J (e-pub ahead of print).
Bussolati B, Grange C, Bruno S, Buttiglieri S, Deregibus MC, Tei L et al. (2006). Neural-cell adhesion molecule (NCAM) expression by immature and tumor-derived endothelial cells favors cell organization into capillary-like structures. Exp Cell Res 312: 913–924.
Carmeliet P . (2005). Angiogenesis in life, disease and medicine. Nature 438: 932–936.
Collino F, Bussolati B, Gerbaudo E, Marozio L, Pelissetto S, Benedetto C et al. (2008). Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol 294: F1185–F1194.
Everaerts W, Nilius B, Owsianik G . (2010). The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 103: 2–17.
Fiorio Pla A, Grange C, Antoniotti S, Tomatis C, Merlino A, Bussolati B et al. (2008). Arachidonic acid-induced Ca2+ entry is involved in early steps of tumor angiogenesis. Mol Cancer Res 6: 535–545.
Fiorio Pla A, Genova T, Pupo E, Tomatis C, Genazzani A, Zaninetti R et al. (2010). Multiple roles of protein kinase A on arachidonic acid-mediated Ca2+ entry and tumor-derived human endothelial cells migration. Mol Cancer Res 8: 1466–1476.
Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH et al. (2005). Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci 25: 2687–2701.
Fiorio Pla A, Munaron L . (2001). Calcium influx, arachidonic acid, and control of endothelial cell proliferation. Cell Calcium 30: 235–244.
Folkman J . (2003). Fundamental concepts of the angiogenic process. Curr Mol Med 3: 643–651.
Gao X, Wu L, O'Neil RG . (2003). Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 278: 27129–27137.
Goswami C, Kuhn J, Heppenstall PA, Hucho T . (2010). Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS ONE 5: e11654.
Grange C, Bussolati B, Bruno S, Fonsato V, Sapino A, Camussi G . (2006). Isolation and characterization of human breast tumor-derived endothelial cells. Oncol Rep 15: 381–386.
Hamdollah Zadeh MA, Glass CA, Magnussen A, Hancox JC, Bates DO . (2008). VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation 15: 605–614.
Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C et al. (2007). Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE 2: e827.
Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC et al. (2004). Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res 64: 8249–8255.
Hyde CA, Missailidis S . (2009). Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int Immunopharmacol 9: 701–715.
Kwan HY, Huang Y, Yao X . (2007). TRP channels in endothelial function and dysfunction. Biochim Biophys Acta 1772: 907–914.
Liedtke W, Kim C . (2005). Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon!. Cell Mol Life Sci 62: 2985–3001.
Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE et al. (2006). A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem 281: 15485–15495.
Ma X, Cao J, Luo J, Nilius B, Huang Y, Ambudkar IS et al. (2010). Depletion of intracellular Ca2+ stores stimulates the translocation of vanilloid transient receptor potential 4-c1 heteromeric channels to the plasma membrane. Arterioscler Thromb Vasc Biol 30: 2249–2255.
Meves H . (2008). Arachidonic acid and ion channels: an update. Br J Pharmacol 155: 4–16.
Moes M, Boonstra J, Regan-Klapisz E . (2010). Novel role of cPLA(2)alpha in membrane and actin dynamics. Cell Mol Life Sci 67: 1547–1557.
Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A . (2004). Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem 279: 25665–25672.
Munaron L, Fiorio Pla A . (2009). Endothelial calcium machinery and angiogenesis: understanding physiology to interfere with pathology. Curr Med Chem 16: 4691–4703.
Mutai H, Mann S, Heller S . (2005). Identification of chicken transmembrane channel-like (TMC) genes: expression analysis in the cochlea. Neuroscience 132: 1115–1122.
Nie D, Honn KV . (2002). Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell Mol Life Sci 59: 799–807.
Nilius B, Owsianik G, Voets T, Peters JA . (2007). Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217.
Patton AM, Kassis J, Doong H, Kohn EC . (2003). Calcium as a molecular target in angiogenesis. Curr Pharm Des 9: 543–551.
Peppelenbosch MP, Qiu RG, de Vries-Smits AM, Tertoolen LG, de Laat SW, McCormick F et al. (1995a). Rac mediates growth factor-induced arachidonic acid release. Cell 81: 849–856.
Peppelenbosch MP, Tertoolen LG, Van der Flier A, De Laat SW . (1995b). Evaluation of single-channel gating kinetics produced after amplitude-based separation of unitary currents. J Neurosci Methods 58: 49–59.
Pocock TM, Foster RR, Bates DO . (2004). Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am J Physiol Heart Circ Physiol 286: H1015–H1026.
Prevarskaya N, Skryma R, Shuba Y . (2010). Ion channels and the hallmarks of cancer. Trends Mol Med 16: 107–121.
Roberts LA, Glenn H, Hahn CS, Jacobson BS . (2003). Cdc42 and RhoA are differentially regulated during arachidonate-mediated HeLa cell adhesion. J Cell Physiol 196: 196–205.
Shin EA, Kim KH, Han SI, Ha KS, Kim JH, Kang KI et al. (1999). Arachidonic acid induces the activation of the stress-activated protein kinase, membrane ruffling and H2O2 production via a small GTPase Rac1. FEBS Lett 452: 355–359.
Singh BB, Lockwich TP, Bandyopadhyay BC, Liu X, Bollimuntha S, Brazer SC et al. (2004). VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell 15: 635–646.
St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E et al. (2000). Genes expressed in human tumor endothelium. Science 289: 1197–1202.
Suzuki M, Hirao A, Mizuno A . (2003). Microtubule-associated [corrected] protein 7 increases the membrane expression of transient receptor potential vanilloid 4 (TRPV4). J Biol Chem 278: 51448–51453.
Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL et al. (2009). TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104: 1123–1130.
Troidl C, Troidl K, Schierling W, Cai WJ, Nef H, Mollmann H et al. (2009). Trpv4 induces collateral vessel growth during regeneration of the arterial circulation. J Cell Mol Med 13: 2613–2621.
Tsai MH, Hall A, Stacey DW . (1989). Inhibition by phospholipids of the interaction between R-ras, rho, and their GTPase-activating proteins. Mol Cell Biol 9: 5260–5264.
Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS . (2005). Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain 1: 17.
Waning J, Vriens J, Owsianik G, Stuwe L, Mally S, Fabian A et al. (2007). A novel function of capsaicin-sensitive TRPV1 channels: involvement in cell migration. Cell Calcium 42: 17–25.
Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P et al. (2002). Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277: 13569–13577.
Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B . (2003). Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438.
Yu PC, Gu SY, Bu JW, Du JL . (2010). TRPC1 is essential for in vivo angiogenesis in zebrafish. Circ Res 106: 1221–1232.
Acknowledgements
This work was supported by the University of Torino, Regione Piemonte (Ricerca Scientifica Applicata Sanita’) and the National Institute of Dental and Craniofacial Research (Intramural Research). We thank Dr Kenneth Yamada (NIDCR, NIH, Bethesda, MD, USA) for the β-actin-mCherry and Dr Michael X. Zhu (Department of Physiology and Cell Biology, Ohio State University, OH, USA) for the TRPV4-GFP construct.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Fiorio Pla, A., Ong, H., Cheng, K. et al. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene 31, 200–212 (2012). https://doi.org/10.1038/onc.2011.231
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.231
Keywords
This article is cited by
-
Inhibition of TRPV4 remodels single cell polarity and suppresses the metastasis of hepatocellular carcinoma
Cell Death & Disease (2023)
-
Transient Receptor Potential Vanilloid 4: a Double-Edged Sword in the Central Nervous System
Molecular Neurobiology (2023)
-
Identification and functional analysis of a biflavone as a novel inhibitor of transient receptor potential vanilloid 4-dependent atherogenic processes
Scientific Reports (2021)
-
Neuropathy-causing TRPV4 mutations disrupt TRPV4-RhoA interactions and impair neurite extension
Nature Communications (2021)
-
Endothelial TRPV4 channels prevent tumor growth and metastasis via modulation of tumor angiogenesis and vascular integrity
Angiogenesis (2021)