Abstract
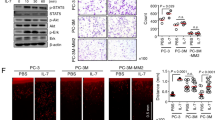
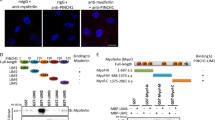
Myopodin is a tumor-suppressor gene that suppresses growth of prostate and urothelial carcinomas. However, the mechanism of myopodin tumor-suppressor activity or signaling that leads to activation of myopodin remains unclear. In this report, we showed that the N-terminus of myopodin binds integrin-linked kinase (ILK) both in vivo and in vitro. An ILK interaction motif of 78 amino acids (amino acids 82–157) was identified in the N-terminus region of myopodin. Induction of ILK-dependent kinase activity by integrin α7 led to phosphorylation of myopodin both in vivo and in vitro. Knocking down ILK dramatically reduced the inhibition of cell growth and motility mediated by myopodin. A mutant of myopodin lacking the ILK interaction motif is inactive in suppressing the growth and motility of PC3 cells. As a result, this study showed a novel and critical signaling pathway that leads to activation of myopodin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Acconcia F, Barnes CJ, Singh RR, Talukder AH, Kumar R . (2007). Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc Natl Acad Sci USA 104: 6782–6787.
Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, Donthamsetty S et al. (2009). Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology 50: 844–851.
Cebrian V, Alvarez M, Aleman A, Palou J, Bellmunt J, Gonzalez-Peramato P et al. (2008). Discovery of myopodin methylation in bladder cancer. J Pathol 216: 111–119.
Deng JT, Van Lierop JE, Sutherland C, Walsh MP . (2001). Ca2+-independent smooth muscle contraction. A novel function for integrin-linked kinase. J Biol Chem 276: 16365–16373.
Donthamsetty S, Bowen W, Mars W, Bhave V, Luo JH, Wu C et al. (2010). Liver specific ablation of integrin linked kinase (ILK) in mice results in enhanced and prolonged cell proliferation and hepatomegaly after phenobarbital administration. Toxicol Sci 113: 358–366.
Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y et al. (2008). Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology 48: 1932–1941.
Han YC, Yu YP, Nelson J, Wu C, Wang H, Michalopoulos GK et al. (2010). Interaction of integrin-linked kinase and miniature chromosome maintenance 7-mediating integrin {alpha}7 induced cell growth suppres. Cancer Res 70: 4375–4384.
Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J et al. (1996). Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379: 91–96.
Jing L, Liu L, Yu YP, Dhir R, Acquafondada M, Landsittel D et al. (2004). Expression of myopodin induces suppression of tumor growth and metastasis. Am J Pathol 164: 1799–1806.
Legate KR, Montanez E, Kudlacek O, Fassler R . (2006). ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7: 20–31.
Lin F, Yu YP, Woods J, Cieply K, Gooding B, Finkelstein P et al. (2001). Myopodin, a synaptopodin homologue, is frequently deleted in invasive prostate cancers. Am J Pathol 159: 1603–1612.
Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D et al. (2001). Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem 276: 27462–27469.
Ren B, Yu YP, Tseng GC, Wu C, Chen K, Rao UN et al. (2007). Analysis of integrin alpha7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcoma. J Natl Cancer Inst 99: 868–880.
Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G et al. (2003). Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev 17: 926–940.
Sanchez-Carbayo M, Schwarz K, Charytonowicz E, Cordon-Cardo C, Mundel P . (2003). Tumor suppressor role for myopodin in bladder cancer: loss of nuclear expression of myopodin is cell-cycle dependent and predicts clinical outcome. Oncogene 22: 5298–5305.
Wu C . (2004). The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim Biophys Acta 1692: 55–62.
Yamaji S, Suzuki A, Sugiyama Y, Koide Y, Yoshida M, Kanamori H et al. (2001). A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J Cell Biol 153: 1251–1264.
Yu YP, Luo JH . (2006). Myopodin-mediated suppression of prostate cancer cell migration involves interaction with zyxin. Cancer Res 66: 7414–7419.
Yu YP, Tseng GC, Luo JH . (2006). Inactivation of myopodin expression associated with prostate cancer relapse. Urology 68: 578–582.
Acknowledgements
This work was supported by grants from National Cancer Institute (R56 CA098249 and RO1 CA098249 to JHL) and American Cancer Society (RSG-08-137-01-CNE to YPY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yu, YP., Luo, JH. Phosphorylation and interaction of myopodin by integrin-link kinase lead to suppression of cell growth and motility in prostate cancer cells. Oncogene 30, 4855–4863 (2011). https://doi.org/10.1038/onc.2011.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.200
Keywords
This article is cited by
-
Prognostic Significance of Promoter Hypermethylation and Diminished Gene Expression of SYNPO2 in Melanoma
Journal of Investigative Dermatology (2015)
-
Myopodin is an F-actin bundling protein with multiple independent actin-binding regions
Journal of Muscle Research and Cell Motility (2013)
-
Diagnostic and prognostic utility of methylation and protein expression patterns of myopodin in colon cancer
Tumor Biology (2012)
-
Mass spectrometry-based phosphoproteomics in cancer research
Frontiers in Biology (2012)