Abstract
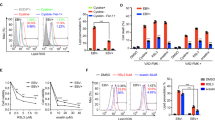
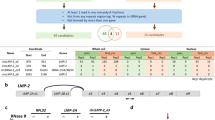
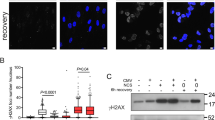
Epstein–Barr virus (EBV) infection is associated with many human neoplasms, in which EBV-derived latent membrane protein-1 (LMP1) appears to be critical, but its exact oncogenic mechanism remains to be defined. To this end, our initial microarray analyses identified a LMP1-inducible gene, Ugene, originally characterized as a binding partner for uracil DNA glycosylase 2, which is highly expressed in malignant colon cancer. In this report, it was found that Ugene, designated herein as LMP1-induced protein (LMPIP), was induced, in a time-dependent manner, in EBV-infected peripheral blood mononuclear cells and LMP1-transfected 293 cells. Functionally, when compared with mock-transfected cells, overexpression of LMPIP in nasopharyngeal carcinoma (NPC) cell lines resulted in a decrease in reactive oxygen species production and maintained mitochondria membrane potential (Δψ) loss induced by H2O2. The NPC cells transfected with LMPIP also showed a decrease in G1 population and an increase in the cell population in sub-G1 and multiploid phase, concomitant with increased levels of cell cycle activators, including cyclin D1 and CDK4. In contrast, silencing of LMPIP expression in the NPC tumor cell lines with short hairpin RNA interference revealed significantly decreased cell population at G1/S phase, while the number of cells in multiploid phase increased. Significantly, NPC cells with LMPIP knock-down also showed a decrease in tumorigenic and transforming activity induced by ectopic LMP1 expression, as determined by analyses of soft agar foci and tumor size in nude mice. Further, elevated LMPIP expression was also noted in cytoplasm and nuclei in EBV-infected NPC tumor cell mass and non-EBV-infected tumor cell lines. These results suggested that LMPIP may have an important mediator role in EBV-mediated neoplasm and may serve as a new target for therapy of tumors induced by EBV infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Ai MD, Li LL, Zhao XR, Wu Y, Gong JP, Cao Y . (2005). Regulation of survivin and CDK4 by Epstein-Barr virus encoded latent membrane protein 1 in nasopharyngeal carcinoma cell lines. Cell Res 15: 777–784.
Bartosz G . (2009). Reactive oxygen species: destroyers or messengers? Biochem Pharmacol 77: 1303–1315.
Behrend L, Henderson G, Zwacka RM . (2003). Reactive oxygen species in oncogenic transformation. Biochem Soc Trans 31: 1441–1444.
Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH . (1991). Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 351: 714–718.
Burhans WC, Heintz NH . (2009). The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic Biol Med 47: 1282–1293.
Chang Y, Sheen TS, Lu J, Huang YT, Chen JY, Yang CS et al. (1998). Detection of transcripts initiated from two viral promoters (Cp and Wp) in Epstein-Barr virus-infected nasopharyngeal carcinoma cells and biopsies. Lab Invest 78: 715–726.
Demetriades C, Mosialos G . (2009). The LMP1 promoter can be transactivated directly by NF-kappaB. J Virol 83: 5269–5277.
Eliopoulos AG, Rickinson AB . (1998). Epstein-Barr virus: LMP1 masquerades as an active receptor. Curr Biol 8: R196–R198.
Eliopoulos AG, Young LS . (2001). LMP1 structure and signal transduction. Semin Cancer Biol 11: 435–444.
Esposito F, Russo L, Chirico G, Ammendola R, Russo T, Cimino F . (2001). Regulation of p21waf1/cip1 expression by intracellular redox conditions. IUBMB Life 52: 67–70.
Fang CY, Lee CH, Wu CC, Chang YT, Yu SL, Chou SP et al. (2009). Recurrent chemical reactivations of EBV promotes genome instability and enhances tumor progression of nasopharyngeal carcinoma cells. Int J Cancer 124: 2016–2025.
Guo C, Zhang X, Fink SP, Platzer P, Wilson K, Willson JK et al. (2008). Ugene, a newly identified protein that is commonly overexpressed in cancer and binds uracil DNA glycosylase. Cancer Res 68: 6118–6126.
Hour TC, Lai YL, Kuan CI, Chou CK, Wang JM, Tu HY et al. (2010). Transcriptional up-regulation of SOD1 by CEBPD: a potential target for cisplatin resistant human urothelial carcinoma cells. Biochem Pharmacol 80: 325–334.
Hsu SH, Hsieh-Li HM, Huang HY, Huang PH, Li H . (2005). bHLH-zip transcription factor Spz1 mediates mitogen-activated protein kinase cell proliferation, transformation, and tumorigenesis. Cancer Res 65: 4041–4050.
Hsu SH, Hsieh-Li HM, Li H . (2004). Dysfunctional spermatogenesis in transgenic mice overexpressing bHLH-Zip transcription factor, Spz1. Exp Cell Res 294: 185–198.
Hsu SH, Shyu HW, Hsieh-Li HM, Li H . (2001). Spz1, a novel bHLH-Zip protein, is specifically expressed in testis. Mech Dev 100: 177–187.
Kanegane H, Nomura K, Miyawaki T, Tosato G . (2002). Biological aspects of Epstein-Barr virus (EBV)-infected lymphocytes in chronic active EBV infection and associated malignancies. Crit Rev Oncol Hematol 44: 239–249.
Kieser A . (2008). Pursuing different ‘TRADDes’: TRADD signaling induced by TNF-receptor 1 and the Epstein-Barr virus oncoprotein LMP1. Biol Chem 389: 1261–1271.
Kis LL, Takahara M, Nagy N, Klein G, Klein E . (2006). IL-10 can induce the expression of EBV-encoded latent membrane protein-1 (LMP-1) in the absence of EBNA-2 in B lymphocytes and in Burkitt lymphoma- and NK lymphoma-derived cell lines. Blood 107: 2928–2935.
Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A et al. (2005). Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res 65: 948–956.
Liebowitz D, Mannick J, Takada K, Kieff E . (1992). Phenotypes of Epstein-Barr virus LMP1 deletion mutants indicate transmembrane and amino-terminal cytoplasmic domains necessary for effects in B-lymphoma cells. J Virol 66: 4612–4616.
Lindahl T, Klein G, Reedman BM, Johansson B, Singh S . (1974). Relationship between Epstein-Barr virus (EBV) DNA and the EBV-determined nuclear antigen (EBNA) in Burkitt lymphoma biopsies and other lymphoproliferative malignancies. Int J Cancer 13: 764–772.
Liu MT, Chang YT, Chen SC, Chuang YC, Chen YR, Lin CS et al. (2005). Epstein-Barr virus latent membrane protein 1 represses p53-mediated DNA repair and transcriptional activity. Oncogene 24: 2635–2646.
Menon SG, Goswami PC . (2007). A redox cycle within the cell cycle: ring in the old with the new. Oncogene 26: 1101–1109.
Menon SG, Sarsour EH, Kalen AL, Venkataraman S, Hitchler MJ, Domann FE et al. (2007). Superoxide signaling mediates N-acetyl-L-cysteine-induced G1 arrest: regulatory role of cyclin D1 and manganese superoxide dismutase. Cancer Res 67: 6392–6399.
Menon SG, Sarsour EH, Spitz DR, Higashikubo R, Sturm M, Zhang H et al. (2003). Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res 63: 2109–2117.
Miller G, Lipman M . (1973). Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci USA 70: 190–194.
Munz C, Moormann A . (2008). Immune escape by Epstein-Barr virus associated malignancies. Semin Cancer Biol 18: 381–387.
Pai S, Khanna R . (2001). Role of LMP1 in immune control of EBV infection. Semin Cancer Biol 11: 455–460.
Queiroga EM, Gualco G, Chioato L, Harrington WJ, Araujo I, Weiss LM et al. (2008). Viral studies in burkitt lymphoma: association with Epstein-Barr virus but not HHV-8. Am J Clin Pathol 130: 186–192.
Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M et al. (2006). Cyclin D1 determines mitochondrial function in vivo. Mol Cell Biol 26: 5449–5469.
Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC . (2009). Redox control of the cell cycle in health and disease. Antioxid Redox Signal 11: 2985–3011.
Sauer H, Wartenberg M, Hescheler J . (2001). Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem 11: 173–186.
Shackelford RE, Kaufmann WK, Paules RS . (1999). Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ Health Perspect 107 (Suppl 1): 5–24.
Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW et al. (1991). Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA 88: 3671–3675.
Soni V, Cahir-McFarland E, Kieff E . (2007). LMP1 TRAFficking activates growth and survival pathways. Adv Exp Med Biol 597: 173–187.
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T . (1995). Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299.
Tanaka H, Matsumura I, Ezoe S, Satoh Y, Sakamaki T, Albanese C et al. (2002). E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol Cell 9: 1017–1029.
Thorley-Lawson DA, Allday MJ . (2008). The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat Rev Microbiol 6: 913–924.
Tsao SW, Tramoutanis G, Dawson CW, Lo AK, Huang DP . (2002). The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol 12: 473–487.
Yu JS, Tsai HC, Wu CC, Weng LP, Li HP, Chung PJ et al. (2002). Induction of inducible nitric oxide synthase by Epstein-Barr virus B95-8-derived LMP1 in Balb/3T3 cells promotes stress-induced cell death and impairs LMP1-mediated transformation. Oncogene 21: 8047–8061.
Zheng H, Li LL, Hu DS, Deng XY, Cao Y . (2007). Role of Epstein-Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol 4: 185–196.
Acknowledgements
We thank Dr SK Huang for critically reviewing this paper. This work was supported in part by Research Grants NSC-97-2311-B-037-002-MY3, NSC-97-2314-B-037-015 and NSC-98-2320-B- 037-026-MY3 from the National Science Council, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Wang, LT., Lin, CS., Chai, CY. et al. Functional interaction of Ugene and EBV infection mediates tumorigenic effects. Oncogene 30, 2921–2932 (2011). https://doi.org/10.1038/onc.2011.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.16
Keywords
This article is cited by
-
A cancer tissue-specific FAM72 expression profile defines a novel glioblastoma multiform (GBM) gene-mutation signature
Journal of Neuro-Oncology (2019)
-
The protein p17 signaling pathways in cancer
Tumor Biology (2013)