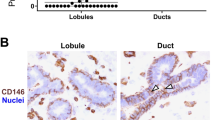
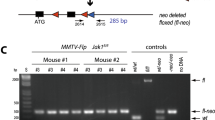
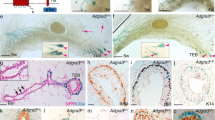
Abstract
Progenitor cells are considered an important cell of origin of human malignancies. However, there has not been any single gene that can define mammary bipotential progenitor cells, and as such it has not been possible to use genetic methods to introduce oncogenic alterations into these cells in vivo to study tumorigenesis from them. Keratin 6a is expressed in a subset of mammary luminal epithelial cells and body cells of terminal end buds. By generating transgenic mice using the Keratin 6a (K6a) gene promoter to express tumor virus A (tva), which encodes the receptor for avian leukosis virus subgroup A (ALV/A), we provide direct evidence that K6a+ cells are bipotential progenitor cells, and the first demonstration of a non-basal location for some biopotential progenitor cells. These K6a+ cells were readily induced to form mammary tumors by intraductal injection of RCAS (an ALV/A-derived vector) carrying the gene encoding the polyoma middle T antigen. Tumors in this K6a-tva line were papillary and resembled the normal breast-like subtype of human breast cancer. This is the first model of this subtype of human tumors and thus may be useful for preclinical testing of targeted therapy for patients with normal-like breast cancer. These observations also provide direct in vivo evidence for the hypothesis that the cell of origin affects mammary tumor phenotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Abbreviations
- K6:
-
keratin 6
- tva:
-
tumor virus A
- MMTV:
-
mouse mammary tumor virus
- PyMT:
-
polyoma middle T antigen
- αSMA:
-
α smooth muscle actin
- RCAS:
-
replication competent
- ALV-LTR:
-
splice acceptor, Bryan-RSV pol, subgroup A virus
References
Andrechek ER, Hardy WR, Laing MA, Muller WJ . (2004). Germ-line expression of an oncogenic erbB2 allele confers resistance to erbB2-induced mammary tumorigenesis. Proc Natl Acad Sci USA 101: 4984–4989.
Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC et al. (2007). Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9: 201–209.
Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A et al. (2009). The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One 4: e6594.
Bai L, Rohrschneider LR . (2010). s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev 24: 1882–1892.
Bates P, Young JA, Varmus HE . (1993). A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74: 1043–1051.
Desai KV, Xiao N, Wang W, Gangi L, Greene J, Powell JI et al. (2002). Initiating oncogenic event determines gene-expression patterns of human breast cancer models. Proc Natl Acad Sci USA 99: 6967–6972.
Dilworth SM . (2002). Polyoma virus middle T antigen and its role in identifying cancer-related molecules. Nat Rev Cancer 2: 951–956.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ et al. (2003). in vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17: 1253–1270.
Du Z, Podsypanina K, Huang H, McGrath A, Toneff MJ, Bogoslovskaia E et al. (2006). Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models. Proc Natl Acad Sci USA 103: 17396–17401.
Fisher GH, Orsulic S, Holland E, Hively WP, Li Y, Lewis BC et al. (1999). Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene 18: 5253–5260.
Grimm SL, Bu W, Longley MA, Roop DR, Li Y, Rosen JM . (2006). Keratin 6 is not essential for mammary gland development. Breast Cancer Res 8: R29.
Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU . (2004). Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene 23: 6980–6985.
Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z et al. (2007). Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol 8: R76.
Hoadley KA, Weigman VJ, Fan C, Sawyer LR, He X, Troester MA et al. (2007). EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics 8: 258.
Hughes SH . (2004). The RCAS vector system. Folia Biol (Praha) 50: 107–119.
Ince TA, Richardson AL, Bell GW, Saitoh M, Godar S, Karnoub AE et al. (2007). Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell 12: 160–170.
Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C et al. (2010). Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell 17: 65–76.
Li Y, Rosen JM . (2005). Stem/progenitor cells in mouse mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia 10: 17–24.
Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X et al. (2003). Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA 100: 15853–15858.
Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH et al. (2009). Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15: 907–913.
Lindvall C, Bu W, Williams BO, Li Y . (2007). Wnt signaling, stem cells, and the cellular origin of breast cancer. Stem Cell Rev 3: 157–168.
Mollet M, Godoy-Silva R, Berdugo C, Chalmers JJ . (2008). Computer simulations of the energy dissipation rate in a fluorescence-activated cell sorter: implications to cells. Biotechnol Bioeng 100: 260–272.
Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R et al. (2010). BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 7: 403–417.
Rosner A, Miyoshi K, Landesman-Bollag E, Xu X, Seldin DC, Moser AR et al. (2002). Pathway pathology: histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am J Pathol 161: 1087–1097.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML et al. (2006). Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88.
Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ . (2006). CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res 8: R7.
Smith GH, Mehrel T, Roop DR . (1990). Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ 1: 161–170.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D et al. (2006). Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997.
Toneff M, Du Z, Dong J, Huang J, Sinai P, Forman J et al. (2010). Somatic expression of PyMT or activated ErbB2 induces estrogen-independent mammary tumorigenesis. Neoplasia 12: 718–726.
Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE . (2008). The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res 68: 7711–7717.
Vargo-Gogola T, Rosen JM . (2007). Modelling breast cancer: one size does not fit all. Nat Rev Cancer 7: 659–672.
Visvader JE . (2011). Cells of origin in cancer. Nature 469: 314–322.
Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA . (2002). Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol 245: 42–56.
Wojcik SM, Longley MA, Roop DR . (2001). Discovery of a novel murine keratin 6 (K6) isoform explains the absence of hair and nail defects in mice deficient for K6a and K6b. J Cell Biol 154: 619–630.
Zeng YA, Nusse R . (2010). Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6: 568–577.
Acknowledgements
We thank Tammy Tong, Amanda McGrath, Ekaterina Bogoslovskaia, Andrei Lazarev, Meghali Goswami and Yiqun Zhang for technical assistance, Drs Jeffrey Rosen and Michael Lewis for stimulating discussions and/or critical review of this manuscript, as well as Drs Robert D Cardiff and Barry Gusterson for advice on pathological comparisons. This work was supported in part by funds from USAMRMC BC030755 and BC073703 (to YL), National Institutes of Health CA113869 and CA124820 (to YL), Project 5 of MMHCC U01 CA084243-07 (to YL; U01 PI: Dr Jeffrey Rosen) and a developmental project of NCI P50 CA058183 (to YL; SPORE PI: Dr C Kent Osborne).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Bu, W., Chen, J., Morrison, G. et al. Keratin 6a marks mammary bipotential progenitor cells that can give rise to a unique tumor model resembling human normal-like breast cancer. Oncogene 30, 4399–4409 (2011). https://doi.org/10.1038/onc.2011.147
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.147
Keywords
This article is cited by
-
Susceptibility of cytoskeletal-associated proteins for tumor progression
Cellular and Molecular Life Sciences (2022)
-
Intraductal Injection of Lentivirus Vectors for Stably Introducing Genes into Rat Mammary Epithelial Cells in Vivo
Journal of Mammary Gland Biology and Neoplasia (2020)
-
Novel K6-K14 keratin fusion enhances cancer stemness and aggressiveness in oral squamous cell carcinoma
Oncogene (2019)
-
Continuous cell supply from Krt7-expressing hematopoietic stem cells during native hematopoiesis revealed by targeted in vivo gene transfer method
Scientific Reports (2017)
-
The PR status of the originating cell of ER/PR-negative mouse mammary tumors
Oncogene (2016)