Abstract
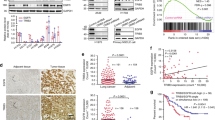
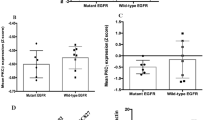
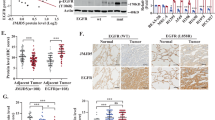
An adaptor protein FRS2β inhibits epidermal growth factor-receptor (EGFR) tyrosine kinase without being phosphorylated at tyrosine residues after EGF stimulation. Although binding to ERK appears to be important for this inhibition, the precise molecular mechanisms and the role of FRS2β in signal transduction mediated by other EGFR family members, as well as its role in human cancer, remain unclear. In this study, we demonstrate that FRS2β inhibits anchorage-independent cell growth induced by oncogenic ErbB2, another member of EGFR family, and that it inhibits heterodimer formation between EGFR and ErbB2. We mapped the residues important for the FRS2β and ERK interaction to two docking (D) domain-like sequences on FRS2β and two aspartic acid residues in the common docking (CD) domain of ERK. Moreover, in response to EGF, ERK translocated to the plasma membrane in cells expressing FRS2β but not an FRS2β mutant in which four arginine residues in the D domains were replaced with alanines, suggesting that FRS2β serves as a plasma membrane anchor for activated ERK. Finally, a low mRNA expression level of FRS2β was significantly correlated with poor prognosis in a cohort of 60 non-small cell lung cancer patients. Therefore, we have identified the molecular mechanisms by which FRS2β acts as a feedback inhibitor of EGFR family members and suggest a role for FRS2β as a tumor suppressor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Akiyama T, Matsuda S, Namba Y, Saito T, Toyoshima K, Yamamoto T . (1991). The transforming potential of the c-erbB2 protein is regulated by its authophosphorylation at the carbodyl-terminal domain. Mol Cell Biol 11: 833–842.
Bargmann CI, Weinberg RA . (1988). Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J 7: 2043–2052.
Baselga J, Arteaga CL . (2005). Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol 23: 2445–2459.
Blume-Jensen P, Hunter T . (2001). Oncogenic kinase signalling. Nature 411: 355–365.
Butch ER, Guan KL . (1996). Characterization of ERK1 activation site mutants and the effect on recognition by MEK1 and MEK2. J Biol Chem 271: 4230–4235.
Eswarakumar VP, Lax I, Schlessinger J . (2005). Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16: 139–149.
Gotoh N . (2008). Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci 99: 1319–1325.
Gotoh N . (2009). Feedback inhibitors of the epidermal growth factor receptor signaling pathways. Int J Biochem Cell Biol 41: 511–515.
Gotoh N, Ito M, Yamamoto S, Yoshino I, Song N, Wang Y et al. (2004a). Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc Natl Acad Sci USA 101: 17144–17149.
Gotoh N, Laks S, Nakashima M, Lax I, Schlessinger J . (2004b). FRS2 family docking proteins with overlapping roles in activation of MAP kinase have distinct spatial-temporal patterns of expression of their transcripts. FEBS Lett 564: 14–18.
Gotoh N, Manova K, Tanaka S, Murohashi M, Hadari Y, Lee A et al. (2005). The docking protein FRS2alpha is an essential component of multiple fibroblast growth factor responses during early mouse development. Mol Cell Biol 25: 4105–4116.
Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J . (2001). Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc Natl Acad Sci USA 98: 8578–8583.
Huang L, Gotoh N, Zhang S, Shibuya M, Yamamoto T, Tsuchida N . (2004). SNT-2 interacts with ERK2 and negatively regulates ERK2 signaling in response to EGF stimulation. Biochem Biophys Res Commun 324: 1011–1017.
Huang L, Watanabe M, Chikamori M, Kido Y, Yamamoto T, Shibuya M et al. (2006). Unique role of SNT-2/FRS2beta/FRS3 docking/adaptor protein for negative regulation in EGF receptor tyrosine kinase signaling pathways. Oncogene 25: 6457–6466.
Hynes NE, Lane HA . (2005). ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5: 341–354.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T et al. (2008). Cancer statistics, 2008. CA Cancer J Clin 58: 71–96.
Kawamura-Tsuzuku J, Suzuki T, Yoshida Y, Yamamoto T . (2004). Nuclear localization of Tob is important for regulation of its antiproliferative activity. Oncogene 23: 6630–6638.
Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T et al. (2003). Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol 31: 1007–1014.
Lax I, Wong A, Lamothe B, Lee A, Frost A, Hawes J et al. (2002). The docking protein FRS2alpha controls a MAP kinase-mediated negative feedback mechanism for signaling by FGF receptors. Mol Cell 10: 709–719.
Lee T, Hoofnagle AN, Kabuyama Y, Stroud J, Min X, Goldsmith EJ et al. (2004). Docking motif interactions in MAP kinases revealed by hydrogen exchange mass spectrometry. Mol Cell 14: 43–55.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S et al. (2004). EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500.
Sato T, Gotoh N . (2009). The FRS2 family of docking/scaffolding adaptor proteins as therapeutic targets of cancer treatment. Expert Opin Ther Targets 13: 689–700.
Schlessinger J . (2000). Cell signaling by receptor tyrosine kinases. Cell 103: 211–225.
Sharrocks AD, Yang SH, Galanis A . (2000). Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem Sci 25: 448–453.
Sonobe M, Manabe T, Wada H, Tanaka F . (2005). Mutations in the epidermal growth factor receptor gene are linked to smoking-independent, lung adenocarcinoma. Br J Cancer 93: 355–363.
Sonobe M, Manabe T, Wada H, Tanaka F . (2006). Lung adenocarcinoma harboring mutations in the ERBB2 kinase domain. J Mol Diagn 8: 351–356.
Tanoue T, Adachi M, Moriguchi T, Nishida E . (2000). A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol 2: 110–116.
Tanoue T, Nishida E . (2003). Molecular recognitions in the MAP kinase cascades. Cell Signal 15: 455–462.
Yamamoto S, Yoshino I, Shimazaki T, Murohashi M, Hevner RF, Lax I et al. (2005). Essential role of Shp2-binding sites on FRS2alpha for corticogenesis and for FGF2-dependent proliferation of neural progenitor cells. Proc Natl Acad Sci USA 102: 15983–15988.
Yarden Y, Sliwkowski MX . (2001). Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137.
Acknowledgements
We are grateful to Dr Toshio Kitamura for valuable reagents for retrovirus expression system and Dr Tadashi Yamamoto for NIH3T3 cells expressing oncogenic ErbB2 and for expression vectors for ErbB2 and VE mutant. This work was supported by grants from the Ministry of Health, Labor and Welfare of Japan for the 3rd-term Comprehensive 10-year Strategy for Cancer Control; from the Naito Foundation; and Cell Science Research Foundation to NG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Iejima, D., Minegishi, Y., Takenaka, K. et al. FRS2β, a potential prognostic gene for non-small cell lung cancer, encodes a feedback inhibitor of EGF receptor family members by ERK binding. Oncogene 29, 3087–3099 (2010). https://doi.org/10.1038/onc.2010.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.69
Keywords
This article is cited by
-
Role and expression of FRS2 and FRS3 in prostate cancer
BMC Cancer (2011)