Abstract
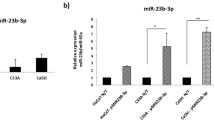
Deregulation of microRNA (miRNA or miR) expression in human cervical cancer is associated frequently with human papillomavirus (HPV) integration. miR-23b is often downregulated in HPV-associated cervical cancer. Interestingly, urokinase-type plasminogen activator (uPA), the miR-23b target, is detected in cervical cancer, but not in normal cervical tissues. Thus, the importance of miR-23b and uPA in HPV-associated cervical cancer development is investigated. In this study, the high-risk subtype HPV-16 E6 oncoprotein was found to decrease the expression of miR-23b, increase the expression of uPA, and thus induce the migration of human cervical carcinoma SiHa and CaSki cells. uPA is the target gene for miR-23b as the miR repressed uPA expression and interacted with the 3′-untranslated region of uPA mRNA. The tumor suppressor p53 is known to be inactivated by HPV-16 E6. A consensus p53 binding site is detected in the promoter region of miR-23b, whereas p53 trans-activated and also interacted with the miR’s promoter. Therefore, p53 is believed to mediate the HPV-16 E6 downregulation of miR-23b. From the above, miR-23b/uPA are confirmed to be involved in HPV-16 E6-associated cervical cancer development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Andreasen PA, Kjoller L, Christensen L, Duffy MJ . (1997). The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72: 1–22.
Bai L, Wei L, Wang J, Li X, He P . (2006). Extended effects of human papillomavirus 16 E6-specific short hairpin RNA on cervical carcinoma cells. Int J Gynecol Cancer 16: 718–729.
Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T et al. (2008). p53-responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res 68: 10094–10104.
Brummelkamp TR, Bernards R, Agami R . (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553.
Calin GA, Croce CM . (2006). MicroRNA signatures in human cancers. Nat Rev Cancer 6: 857–866.
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S et al. (2004). Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 101: 2999–3004.
Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH et al. (2007). Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26: 745–752.
Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH . (2008). Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev 34: 122–136.
Davis BN, Hata A . (2009). Regulation of microRNA biogenesis: a miRiad of mechanisms. Cell Commun Signal 7: 18.
Diederichs S, Haber DA . (2006). Sequence variations of microRNAs in human cancer: alterations in predicted secondary structure do not affect processing. Cancer Res 66: 6097–6104.
Duffy MJ, Maguire TM, McDermott EW, O'Higgins N . (1999). Urokinase plasminogen activator: a prognostic marker in multiple types of cancer. J Surg Oncol 71: 130–135.
Esquela-Kerscher A, Slack FJ . (2006). Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6: 259–269.
Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM . (2006). microRNA expression and function in cancer. Trends Mol Med 12: 580–587.
Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ et al. (2007). A microRNA signature of hypoxia. Mol Cell Biol 27: 1859–1867.
Kunz C, Pebler S, Otte J, von der Ahe D . (1995). Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res 23: 3710–3717.
Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ, Hwang SY et al. (2008). Altered microRNA expression in cervical carcinomas. Clin Cancer Res 14: 2535–2542.
Lui WO, Pourmand N, Patterson BK, Fire A . (2007). Patterns of known and novel small RNAs in human cervical cancer. Cancer Res 67: 6031–6043.
Martinez I, Gardiner AS, Board KF, Monzon FA, Edwards RP, Khan SA . (2008). Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene 27: 2575–2582.
Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV et al. (2003). Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348: 518–527.
Muralidhar B, Goldstein LD, Ng G, Winder DM, Palmer RD, Gooding EL et al. (2007). Global microRNA profiles in cervical squamous cell carcinoma depend on drosha expression levels. J Pathol 212: 368–377.
Riethdorf L, Riethdorf S, Petersen S, Bauer M, Herbst H, Janicke F et al. (1999). Urokinase gene expression indicates early invasive growth in squamous cell lesions of the uterine cervix. J Pathol 189: 245–250.
Salvi A, Sabelli C, Moncini S, Venturin M, Arici B, Riva P et al. (2009). MicroRNA-23b mediates urokinase and c-met downmodulation and a decreased migration of human hepatocellular carcinoma cells. FEBS J 276: 2966–2982.
Scaria V, Jadhav V . (2007). microRNAs in viral oncogenesis. Retrovirology 4: 82.
Shetty P, Velusamy T, Bhandary YP, Shetty RS, Liu MC, Shetty S . (2008). Urokinase expression by tumor suppressor protein p53: a novel role in mRNA turnover. Am J Respir Cell Mol Biol 39: 364–372.
Soliman PT, Slomovitz BM, Wolf JK . (2004). Mechanisms of cervical cancer. Drug Discovery Today 1: 253–258.
Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A et al. (2007). Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: MiR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 6: 1586–1593.
Turner MA, Palefsky JM . (1995). Urokinase plasminogen activator expression by primary and HPV 16-transformed keratinocytes. Clin Exp Metast 13: 260–268.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV et al. (1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189: 12–19.
Wang X, Wang HK, McCoy JP, Banerjee NS, Rader JS, Broker TR et al. (2009). Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA 15: 637–647.
Wu W, Zou M, Brickley DR, Pew T, Conzen SD . (2006). Glucocorticoid receptor activation signals through forkhead transcription factor 3a in breast cancer cells. Mol Endocrinol 20: 2304–2314.
Zhang B, Pan X, Cobb GP, Anderson TA . (2007). microRNAs as oncogenes and tumor suppressors. Dev Biol 302: 1–12.
Zuna RE, Allen RA, Moore WE, Mattu R, Dunn ST . (2004). Comparison of human papillomavirus genotypes in high-grade squamous intraepithelial lesions and invasive cervical carcinoma: evidence for differences in biologic potential of precursor lesions. Mod Pathol 17: 1314–1322.
zur Hausen H . (2002). Papillomaviruses and cancer: From basic studies to clinical application. Nat Rev Cancer 2: 342–350.
Acknowledgements
The study is supported by grants from Hong Kong Research Grants and Council Earmarked Grants 466908, 467609.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Au Yeung, C., Tsang, T., Yau, P. et al. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene 30, 2401–2410 (2011). https://doi.org/10.1038/onc.2010.613
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.613
Keywords
This article is cited by
-
MicroRNA regulation of phenotypic transformations in vascular smooth muscle: relevance to vascular remodeling
Cellular and Molecular Life Sciences (2023)
-
Comprehensive analysis of differentially expressed circRNAs and ceRNA regulatory network in porcine skeletal muscle
BMC Genomics (2021)
-
Cervical cancer development, chemoresistance, and therapy: a snapshot of involvement of microRNA
Molecular and Cellular Biochemistry (2021)
-
miR-34a-5p Inhibits Cell Proliferation, Migration and Invasion Through Targeting JAG1/Notch1 Pathway in HPV-Infected Human Epidermal Keratinocytes
Pathology & Oncology Research (2020)
-
MiR-23b-3p reduces the proliferation, migration and invasion of cervical cancer cell lines via the reduction of c-Met expression
Scientific Reports (2020)