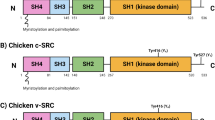
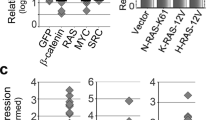
Abstract
Pancreas cancer is one of the most lethal malignancies and is characterized by activating mutations of Kras, present in 95% of patients. More than 60% of pancreatic cancers also display increased c-Src activity, which is associated with poor prognosis. Although loss of tumor suppressor function (for example, p16, p53, Smad4) combined with oncogenic Kras signaling has been shown to accelerate pancreatic duct carcinogenesis, it is unclear whether elevated Src activity contributes to Kras-dependent tumorigenesis or is simply a biomarker of disease progression. Here, we demonstrate that in the context of oncogenic Kras, activation of c-Src through deletion of C-terminal Src kinase (CSK) results in the development of invasive pancreatic ductal adenocarcinoma (PDA) by 5–8 weeks. In contrast, deletion of CSK alone fails to induce neoplasia, while oncogenic Kras expression yields PDA at low frequency after a latency of 12 months. Analysis of cell lines derived from Ras/Src-induced PDA's indicates that oncogenic Ras/Src cooperativity may lead to genomic instability, yet Ras/Src-driven tumor cells remain dependent on Src signaling and as such, Src inhibition suppresses growth of Ras/Src-driven tumors. These findings demonstrate that oncogenic Ras/Src cooperate to accelerate PDA onset and support further studies of Src-directed therapies in pancreatic cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J et al. (2003). Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev 17: 3112–3126.
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M . (1988). Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53: 549–554.
Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P et al. (2006). Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev 20: 3130–3146.
Barton CM, Hall PA, Hughes CM, Gullick WJ, Lemoine NR . (1991). Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J Pathol 163: 111–116.
Billadeau DD, Chatterjee S, Bramati P, Sreekumar R, Shah V, Hedin K et al. (2006). Characterization of the CXCR4 signaling in pancreatic cancer cells. Int J Gastrointest Cancer 37: 110–119.
Bromann PA, Korkaya H, Courtneidge SA . (2004). The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23: 7957–7968.
Broome MA, Courtneidge SA . (2000). No requirement for src family kinases for PDGF signaling in fibroblasts expressing SV40 large T antigen. Oncogene 19: 2867–2869.
Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N et al. (2009). An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med 15: 1163–1169.
Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA . (1999). Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 4: 915–924.
Fanidi A, Harrington EA, Evan GI . (1992). Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature 359: 554–556.
Fenech M . (2000). The in vitro micronucleus technique. Mutat Res 455: 81–95.
Grimm J, Potthast A, Wunder A, Moore A . (2003). Magnetic resonance imaging of the pancreas and pancreatic tumors in a mouse orthotopic model of human cancer. Int J Cancer 106: 806–811.
Hall PA, Hughes CM, Staddon SL, Richman PI, Gullick WJ, Lemoine NR . (1990). The c-erb B-2 proto-oncogene in human pancreatic cancer. J Pathol 161: 195–200.
Hernandez-Munoz I, Skoudy A, Real FX, Navarro P . (2008). Pancreatic ductal adenocarcinoma: cellular origin, signaling pathways and stroma contribution. Pancreatology 8: 462–469.
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA . (2006). Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 20: 1218–1249.
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA et al. (2003). Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4: 437–450.
Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH et al. (2005). Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7: 469–483.
Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV et al. (2004). An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 28: 977–987.
Hruban RH, Wilentz RE, Kern SE . (2000). Genetic progression in the pancreatic ducts. Am J Pathol 156: 1821–1825.
Hunter T, Sefton BM . (1980). Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA 77: 1311–1315.
Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S et al. (2006). Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev 20: 3147–3160.
Irby RB, Yeatman TJ . (2000). Role of Src expression and activation in human cancer. Oncogene 19: 5636–5642.
Ishizawar R, Parsons SJ . (2004). c-Src and cooperating partners in human cancer. Cancer Cell 6: 209–214.
Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM et al. (2007). Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell 11: 229–243.
Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R et al. (2001). Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15: 3243–3248.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ . (2009). Cancer statistics, 2009. CA Cancer J Clin 59: 225–249.
Kabil A, Silva E, Kortenkamp A . (2008). Estrogens and genomic instability in human breast cancer cells—involvement of Src/Raf/Erk signaling in micronucleus formation by estrogenic chemicals. Carcinogenesis 29: 1862–1868.
Kasahara K, Nakayama Y, Nakazato Y, Ikeda K, Kuga T, Yamaguchi N . (2007). Src signaling regulates completion of abscission in cytokinesis through ERK/MAPK activation at the midbody. J Biol Chem 282: 5327–5339.
Kline CL, Jackson R, Engelman R, Pledger WJ, Yeatman TJ, Irby RB . (2008). Src kinase induces tumor formation in the c-SRC C57BL/6 mouse. Int J Cancer 122: 2665–2673.
Koreckij T, Nguyen H, Brown LG, Yu EY, Vessella RL, Corey E . (2009). Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer 101: 263–268.
Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K et al. (2004). Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 47: 6658–6661.
Lutz MP, Esser IB, Flossmann-Kast BB, Vogelmann R, Luhrs H, Friess H et al. (1998). Overexpression and activation of the tyrosine kinase src in human pancreatic carcinoma. Biochem Biophys Res Commun 243: 503–508.
Maddalena AS, Hainfellner JA, Hegi ME, Glatzel M, Aguzzi A . (1999). No complementation between TP53 or RB-1 and v-src in astrocytomas of GFAP-v-src transgenic mice. Brain Pathol 9: 627–637.
Martin GS . (2001). The hunting of the src. Nat Rev Mol Cell Biol 2: 467–475.
Matsumoto T, Kiguchi K, Jiang J, Carbajal S, Ruffino L, Beltran L et al. (2004). Development of transgenic mice that inducibly express an active form of c-src in the epidermis. Mol Carcinog 40: 189–200.
Morton JP, Karim SA, Graham K, Timpson P, Jamieson N, Athineos D et al. (2010). Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 139: 292–303.
Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K et al. (2008). Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci USA 105: 9343–9348.
Podsypanina K, Politi K, Beverly LJ, Varmus HE . (2008). Oncogene cooperation in tumor maintenance and tumor recurrence in mouse mammary tumors induced by Myc and mutant Kras. Proc Natl Acad Sci USA 105: 5242–5247.
Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD et al. (2009). Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer Res 69: 1383–1391.
Rous P . (1911). A sarcoma of the fowl transmissable by an agent separable from the tumor cells. J Exp Med 13: 397–411.
Rucci N, Recchia I, Angelucci A, Alamanou M, Del Fattore A, Fortunati D et al. (2006). Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther 318: 161–172.
Schmedt C, Saijo K, Niidome T, Kuhn R, Aizawa S, Tarakhovsky A . (1998). Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature 394: 901–904.
Stehelin D, Varmus HE, Bishop JM, Vogt PK . (1976). DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature 260: 170–173.
Talamonti MS, Roh MS, Curley SA, Gallick GE . (1993). Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest 91: 53–60.
Thomas RM, Kim J, Revelo-Penafiel MP, Angel R, Dawson DW, Lowy AM . (2008). The chemokine receptor CXCR4 is expressed in pancreatic intraepithelial neoplasia. Gut 57: 1555–1560.
Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA et al. (2007). The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res 67: 6075–6082.
Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY et al. (2006). Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol 168: 962–972.
Vidal M, Larson DE, Cagan RL . (2006). Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell 10: 33–44.
Vitali R, Mancini C, Cesi V, Tanno B, Piscitelli M, Mancuso M et al. (2009). Activity of tyrosine kinase inhibitor Dasatinib in neuroblastoma cells in vitro and in orthotopic mouse model. Int J Cancer 125: 2547–2555.
Warshaw AL, Fernandez-del Castillo C . (1992). Pancreatic carcinoma. N Engl J Med 326: 455–465.
Yezhelyev MV, Koehl G, Guba M, Brabletz T, Jauch KW, Ryan A et al. (2004). Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res 10: 8028–8036.
Acknowledgements
We thank Charles Yi and Dana Wu for excellent technical assistance. We acknowledge Richard Aspinall, Lisette Acevedo, Josh Greenberg and David Tuveson for valuable discussions, Greg Boivin for histological analysis, as well as Richard Levenson and Kristin Lane for NuBrio imaging. This work was supported by NIH grants R21CA104898, P01-CA078045 (DAC) and Collaborative Translational Research grants from Moores UCSD Cancer Center (DAC, DJS and AML).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Shields, D., Murphy, E., Desgrosellier, J. et al. Oncogenic Ras/Src cooperativity in pancreatic neoplasia. Oncogene 30, 2123–2134 (2011). https://doi.org/10.1038/onc.2010.589
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.589
Keywords
This article is cited by
-
Ex vivo organotypic cultures for synergistic therapy prioritization identify patient-specific responses to combined MEK and Src inhibition in colorectal cancer
Nature Cancer (2022)
-
p27kip1 expression and phosphorylation dictate Palbociclib sensitivity in KRAS-mutated colorectal cancer
Cell Death & Disease (2021)
-
Rho guanine nucleotide exchange factor ARHGEF10 is a putative tumor suppressor in pancreatic ductal adenocarcinoma
Oncogene (2020)
-
Novel regulation of Ras proteins by direct tyrosine phosphorylation and dephosphorylation
Cancer and Metastasis Reviews (2020)
-
Overexpression and Tyr421-phosphorylation of cortactin is induced by three-dimensional spheroid culturing and contributes to migration and invasion of pancreatic ductal adenocarcinoma (PDAC) cells
Cancer Cell International (2019)