Abstract
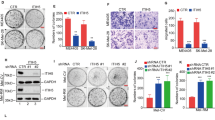
FOXO transcription factors are evolutionarily conserved proteins that orchestrate gene expression programs known to control a variety of cellular processes such as cell cycle, apoptosis, DNA repair and protection from oxidative stress. As the abrogation of FOXO function is a key feature of many tumor cells, regulation of FOXO factors is receiving increasing attention in cancer research. In order to discover genes involved in the regulation of FOXO activity, we performed a large-scale RNA-mediated interference (RNAi) screen using cell-based reporter systems that monitor transcriptional activity and subcellular localization of FOXO. We identified genes previously implicated in phosphoinositide 3-kinase/Akt signaling events, which are known to be important for FOXO function. In addition, we discovered a previously unrecognized FOXO-repressor function of TRIB2, the mammalian homolog of the Drosophila gene tribbles. A cancer-profiling array revealed specific overexpression of TRIB2 in malignant melanoma, but not in other types of skin cancer. We provide experimental evidence that TRIB2 transcript levels correlate with the degree of cytoplasmic localization of FOXO3a. Moreover, we show that TRIB2 is important in the maintenance of the oncogenic properties of melanoma cells, as its silencing reduces cell proliferation, colony formation and wound healing. Tumor growth was also substantially reduced upon RNAi-mediated TRIB2 knockdown in an in vivo melanoma xenograft model. Our studies suggest that TRIB2 provides the melanoma cells with growth and survival advantages through the abrogation of FOXO function. Altogether, our results show the potential of large-scale cell-based RNAi screens to identify promising diagnostic markers and therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M et al. (2004). A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428: 431–437.
Birmingham A, Anderson EM, Reynolds A, Ilsley-Tyree D, Leake D, Fedorov Y et al. (2006). 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods 3: 199–204.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS et al. (1999). Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868.
Calnan DR, Brunet A . (2008). The FoxO code. Oncogene 27: 2276–2288.
Dansen TB, Burgering BM . (2008). Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol 18: 421–429.
Gomes AR, Brosens JJ, Lam EW . (2008). Resist or die: FOXO transcription factors determine the cellular response to chemotherapy. Cell Cycle 7: 3133–3136.
Grosshans J, Wieschaus E . (2000). A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 101: 523–531.
Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW et al. (2005). The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet 37: 1047–1054.
Hegedus Z, Czibula A, Kiss-Toth E . (2006). Tribbles: novel regulators of cell function; evolutionary aspects. Cell Mol Life Sci 63: 1632–1641.
Hegedus Z, Czibula A, Kiss-Toth E . (2007). Tribbles: a family of kinase-like proteins with potent signalling regulatory function. Cell Signal 19: 238–250.
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J . (2007). qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19.
Hilmi C, Larribere L, Deckert M, Rocchi S, Giuliano S, Bille K et al. (2008). Involvement of FKHRL1 in melanoma cell survival and death. Pigment Cell Melanoma Res 21: 139–146.
Hou L, Panthier JJ, Arnheiter H . (2000). Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: interactions between KIT and MITF. Development 127: 5379–5389.
Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY et al. (2004). IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117: 225–237.
Huang H, Tindall DJ . (2007). Dynamic FoxO transcription factors. J Cell Sci 120: 2479–2487.
Hui RC, Francis RE, Guest SK, Costa JR, Gomes AR, Myatt SS et al (2008). Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther 7: 670–678.
Keeshan K, He Y, Wouters BJ, Shestova O, Xu L, Sai H et al (2006). Tribbles homolog 2 inactivates C/EBPalpha and causes acute myelogenous leukemia. Cancer Cell 10: 401–411.
Mata J, Curado S, Ephrussi A, Rorth P . (2000). Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 101: 511–522.
Meier F, Busch S, Lasithiotakis K, Kulms D, Garbe C, Maczey E et al (2007). Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. Br J Dermatol 156: 1204–1213.
Myatt SS, Lam EW . (2007). The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 7: 847–859.
Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z et al (2007). FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128: 309–323.
Rosado A, Zanella F, Garcia B, Carnero A, Link W . (2008). A dual-color fluorescence-based platform to identify selective inhibitors of Akt signaling. PLoS ONE 3: e1823.
Segura MF, Hanniford D, Menendez S, Reavie L, Zou X, Alvarez-Diaz S et al (2009). Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proc Natl Acad Sci USA 106: 1814–1819.
Soengas MS, Lowe SW . (2003). Apoptosis and melanoma chemoresistance. Oncogene 22: 3138–3151.
Yang H, Zhao R, Yang HY, Lee MH . (2005). Constitutively active FOXO4 inhibits Akt activity, regulates p27 Kip1 stability, and suppresses HER2-mediated tumorigenicity. Oncogene 24: 1924–1935.
Zanella F, Rosado A, Blanco F, Henderson BR, Carnero A, Link W . (2007). An HTS approach to screen for antagonists of the nuclear export machinery using high content cell-based assays. Assay Drug Dev Technol 5: 333–341.
Zanella F, Rosado A, Garcia B, Carnero A, Link W . (2008). Chemical genetic analysis of FOXO nuclear-cytoplasmic shuttling by using image-based cell screening. Chembiochem 9: 2229–2237.
Zanella F, Rosado A, Garcia B, Carnero A, Link W . (2009). Using multiplexed regulation of luciferase activity and GFP translocation to screen for FOXO modulators. BMC Cell Biol 10: 14.
Acknowledgements
This work was supported by a grant from the Spanish MEC (project BIO2006-02432). FZ is the recipient of a Marie Curie Fellowship. We acknowledge the expert technical assistance of D Megias, J C Cigudosa, J Monsech and O Dominguez. We thank M Hu, T Unterman and E Kiss-Toth for providing plasmids and JF Martinez for helpful discussions and critical reading of this paper. We are indebted to R Bernards for advice and for providing us with the NKI shRNA library.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zanella, F., Renner, O., García, B. et al. Human TRIB2 is a repressor of FOXO that contributes to the malignant phenotype of melanoma cells. Oncogene 29, 2973–2982 (2010). https://doi.org/10.1038/onc.2010.58
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.58
Keywords
This article is cited by
-
Therapeutic strategies targeting FOXO transcription factors
Nature Reviews Drug Discovery (2021)
-
TRIB2 functions as novel oncogene in colorectal cancer by blocking cellular senescence through AP4/p21 signaling
Molecular Cancer (2018)
-
TRIB2 regulates the differentiation of MLL–TET1 transduced myeloid progenitor cells
Journal of Molecular Medicine (2018)
-
Trib2 regulates the pluripotency of embryonic stem cells and enhances reprogramming efficiency
Experimental & Molecular Medicine (2017)
-
TRIB2 confers resistance to anti-cancer therapy by activating the serine/threonine protein kinase AKT
Nature Communications (2017)