Abstract
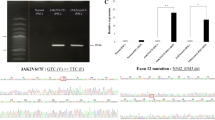
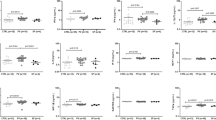
The V617F activating mutation of janus kinase 2 (JAK2), a kinase essential for cytokine signalling, characterizes Polycythemia vera (PV), one of the myeloproliferative neoplasms (MPN). However, not all MPNs carry mutations of JAK2, and in JAK2-mutated patients, expression of JAK2V617F does not always result in clone expansion. In the present study, we provide evidence that inflammation-linked cytokines are required for the growth of JAK2V617F-mutated erythroid progenitors. In a first series of experiments, we searched for cytokines over-expressed in PV using cytokine antibody (Ab) arrays, and enzyme-linked immunosorbent assays for analyses of serum and bone marrow (BM) plasma, and quantitative reverse transcription–PCRs for analyses of cells purified from PV patients and controls. We found that PV patients over-expressed anti-inflammatory hepatocyte growth factor (HGF) and interleukin-11 (IL-11), BM mesenchymal stromal cells (BMMSCs) and erythroblasts being the main producers. In a second series of experiments, autocrine/paracrine cytokine stimulation of erythroblasts was blocked using neutralizing Abs specific for IL-11 or c-MET, the HGF receptor. The growth of JAK2V617F-mutated HEL cells and PV erythroblasts was inhibited, indicating that JAK2-mutated cells depend on HGF and IL-11 for their growth. Additional experiments showed that transient expression of JAK2V617F in BaF-3/erythropoietin receptor cells, and invalidation of JAK2V617F in HEL cells using anti-JAK2 small interfering RNA, did not affect HGF and IL-11 expression. Thus, anti-inflammatory HGF and IL-11 are upregulated in PV and their overproduction is not a consequence of JAK2V617F. As both cytokines contribute to the proliferation of PV erythroblasts, blocking the c-MET/HGF/IL-11 pathways could be of interest as an additional therapeutic option in PV.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aman MJ, Bug G, Aulitzky WE, Huber C, Peschel C . (1996). Inhibition of interleukin-11 by interferon-alpha in human bone marrow stromal cells. Exp Hematol 24: 863–867.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Cancer Genome Project et al. (2005). Acquired mutation of the tyrosine kinase JAK-2 in human myeloproliferative disorders. Lancet 365: 1054–1061.
Bernet A, Mehlen P . (2007). Dependence receptors: when apoptosis controls tumor progression. Bull Cancer 94: E12–E17.
Cleyrat C, Jelinek J, Girodon F, Boissinot M, Ponge T, Harousseau J-L et al. (2010). JAK2 mutation and disease phenotype: A double L611V/V617F in cis mutation of JAK2 is associated with isolated erythrocytosis and increased activation of AKT and ERK1/2 rather than STAT5. Leukemia 24: 1069–1073.
Comoglio PM, Giordano S, Trusolino L . (2008). Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 7: 504–516.
Coppo P, Dusanter-Fourt I, Vainchenker W, Turhan AG . (2009). BCR-ABL induces opposite phenotypes in murine ES cells according to STAT3 activation levels. Cell Signal 21: 52–60.
Corre-Buscail I, Pineau D, Boissinot M, Hermouet S . (2005). Erythropoietin-independent erythroid colony formation by bone marrow progenitors exposed to interleukins 11 and 8. Exp Hematol 33: 1299–1308.
Derksen PW, de Gorter DJ, Meijer HP, Bende RJ, van Dijk M, Lokhorst HM et al. (2003). The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia 17: 764–774.
Dobo I, Donnard M, Girodon F, Mossuz P, Boiret N, Boukhari R et al. (2004). Standardization and comparison of endogenous erythroid colony assay performed with bone marrow or blood progenitors for the diagnosis of polycythaemia vera. Hematol J 5: 161–167.
Du W, Hattori Y, Yamada T, Matsumoto K, Nakamura T, Sagawa M et al. (2007). NK4, an antagonist of hepatocyte growth factor (HGF), inhibits growth of multiple myeloma cells: molecular targeting of angiogenic growth factor. Blood 109: 3042–3049.
Ferguson LR, Han DY, Fraser AG, Huebner C, Lam WJ, Morgan AR et al. (2010). Genetic factors in chronic inflammation: Single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn’ disease in a New Zealand population. Mutat Res 690: 108–115.
Geissler K, Ohler L, Fodinger M, Kabrna E, Kollars M, Skoupy S et al. (1998). Interleukin-10 inhibits erythropoietin-independent growth of erythroid bursts in patients with polycythemia vera. Blood 92: 1967–1972.
Girodon F, Schaeffer C, Cleyrat C, Mounier M, Lafont I, Dos Santos F et al. (2008). Frequent reduction or absence of detection of the JAK2-mutated clone in JAK2V617F-positive patients within the first years of hydroxurea therapy. Haematologica 93: 1723–1727.
Giuliani N, Colla S, Morandi F, Rizzoli V . (2004). The RANK/RANK ligand system is involved in interleukin-6 and interleukin-11 up-regulation by human myeloma cells in the bone marrow microenvironment. Haematologica 89: 1118–1123.
Hermouet S, Godard A, Pineau D, Corre I, Raher S, Lippert E et al. (2002). Abnormal production of interleukin (IL)-11 and IL-8 in polycythemia vera. Cytokine 20: 178–183.
Hitoshi Y, Lin N, Payan DG, Markovtsov V . (2010). The current status and the future of JAK2 inhibitors for the treatment of myeloproliferative diseases. Int J Hematol 91: 189–200.
Ho CL, Lasho TL, Butterfield JH, Tefferi A . (2007). Global cytokine analysis in myeloproliferative disorders. Leuk Res 31: 1389–1392.
Ishii T, Zhao Y, Shi J, Sozer S, Hoffman R, Xu M . (2007). T cells from patients with polycythemia vera elaborate growth factors which contribute to endogenous erythroid and megakaryocyte colony formation. Leukemia 21: 2433–2441.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C et al. (2005). A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434: 1144–1148.
Jenkins BJ, Roberts AW, Najdovska M, Grail D, Ernst M . (2005). The threshold of gp130-dependent STAT3 signalling is critical for normal regulation of hematopoiesis. Blood 105: 3512–3520.
Jenkins BJ, Roberts AW, Greenhill CJ, Najdovska M, Lundgren-May T, Robb L et al. (2007). Pathologic consequences of STAT3 hyperactivation by IL-6 and IL-11 during hematopoiesis and lymphopoiesis. Blood 109: 2380–2388.
Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL et al. (2009). JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet 41: 446–449.
Kiladjian JJ, Cassinat B, Turlure P, Cambier N, Roussel M, Bellucci S et al. (2006). High molecular response rate of polycythemia vera patients treated with pegylated interferon alpha-2a. Blood 108: 2037–2040.
Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL et al. (2009). A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet 41: 455–459.
Kitajima Y, Ide T, Ohtsuka T, Miyazaki K . (2008). Induction of hepatocyte growth factor activator gene expression under hypoxia activates the hepatocyte growth factor/c-Met system via hypoxia inducible factor-1 in pancreatic cancer. Cancer Sci 99: 1341–1347.
Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S et al. (2007). Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res 67: 8615–8623.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. (2005). A gain-of-function mutation of JAK-2 in myeloproliferative disorders. New Engl J Med 352: 1779–1790.
Kralovics R . (2008). Genetic complexity of myeloproliferative neoplasms. Leukemia 22: 1841–1848.
Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL . (2006). JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood 108: 1652–1660.
Lambert JR, Everington T, Linch DC, Gale RE . (2009). In essential thrombocythemia, multiple JAK2-V617F clones are present in most mutant-positive patients: a new disease paradigm. Blood 114: 3018–3023.
Le Bousse-Kerdilés MC, Martyré MC . (1999). Dual implication of fibrogenic cytokines in the pathogenesis of fibrosis and myeloproliferation in myeloid metaplasia with myelofibrosis. Ann Hematol 78: 437–444 . Review.
Lippert E, Boissinot M, Kralovics R, Girodon F, Dobo I, Praloran V et al. (2006). The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood 108: 1865–1867.
Lippert E, Girodon F, Hammond E, Reading NS, Jelinek J, Badbaran A et al. (2009). Concordance of assays designed for the quantitation of JAK2V617F (1849G>T): a multi-centre study. Haematologica 94: 38–45.
Matsuda-Hashii Y, Takai K, Ohta H, Fujisaki H, Tokimasa S, Osugi Y et al. (2004). Hepatocyte growth factor plays roles in the induction and autocrine maintenance of bone marrow stromal cell IL-11, SDF-1 alpha, and stem cell factor. Exp Hematol 32: 955–961.
Musolino C, Calabro L, Bellomo G, Martello F, Loteta B, Pezzano C et al. (2002). Soluble angiogenic factors: implications for chronic myeloproliferative disorders. Am J Hematol 69: 159–163.
Najfeld V, Cozza A, Berkofsy-Fessler W, Prchal J, Scalise A . (2007). Numerical gain and structural rearrangements of JAK2, identified by FISH, characterize both JAK2617V>F-positive and -negative patients with Ph-negative MPD, myelodysplasia, and B-lymphoid neoplasms. Exp Hematol 35: 1668–7166.
Nussenzveig RH, Swierczek SI, Jelinek J, Gaikwad A, Liu E, Verstovsek S et al. (2007). Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol 35: 32–38.
Olcaydu D, Harutyunyan A, Jäger R, Berg T, Gisslinger B, Pabinger I et al. (2009). A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet 41: 450–454.
Panteli KE, Hatzimichael EC, Bouranta PK, Katsaraki A, Seferiadis K, Stebbing J et al. (2005). Serum interleukin (IL)-1, IL-2, sIL-2Ra, IL-6 and thrombopoietin levels in patients with chronic myeloproliferative diseases. British J Haematol 130: 709–715.
Quesniaux VF, Clark SC, Turner K, Fagg B . (1992). Interleukin-11 stimulates multiple phases of erythropoiesis in vitro. Blood 80: 1218–1223.
Radaeva S, Jaruga B, Hong F, Kim WH, Fan S, Cai H et al. (2002). Interferon-alpha activates multiple STAT signals and down-regulates c-Met in primary human hepatocytes. Gastroenterology 122: 1020–1034.
Sattler M, Salgia R . (2007). c-MET and hepatocyte growth factor: potential new targets in cancer therapy. Curr Oncol Rep 9: 102–108.
Schaub FX, Jäger R, Looser R, Hao-Shen H, Hermouet S, Girodon F et al. (2009). Clonal analysis of deletions on chromosome 20q and JAK2-V617F in MPD suggests that del20q acts independently and is not one of the pre-disposing mutations for JAK2-V617F. Blood 113: 2022–2027.
Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ . (1999). Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia 13: 1307–1315.
Shinomiya N, Gao CF, Xie Q, Gustafson M, Waters DJ, Zhang YW et al. (2004). RNA interference reveals that ligand-independent MET activity is required for tumor cell signalling and survival. Cancer Res 64: 7962–7970.
Stellrecht CM, Phillip CJ, Cervantes-Gomez F, Gandhi V . (2007). Multiple myeloma cell killing by depletion of the MET receptor tyrosine kinase. Cancer Res 67: 9913–9920.
Tacchini L, Dansi P, Matteucci E, Desiderio MA . (2001). Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis 22: 1363–1371.
Tefferi A . (2010). JAK inhibitors in myeloproliferative neoplasms. The Hematologist 7: 5 . Review.
Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J et al. (2008). Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood 111: 3931–3940.
Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC . (1993). Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood 82: 3712–3720.
Vardiman JW, Harris NL, Brunning RD . (2002). The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100: 2292–2302.
Verstovsek S . (2010). Therapeutic potential of janus-activated Kinase-2 inhibitors for the management of myelofibrosis. Clin Cancer Res 16: 1988–1996.
Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG . (2006). Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 107: 4274–4281.
Wickenhauser C, Thiele J, Lorenzen J, Schmitz B, Frimpong S, Schramm K et al. (1999). Polycythemia vera megakaryocytes but not megakaryocytes from normal controls and patients with smoker's polycythemia spontaneously express IL-6 and IL-6R and secrete IL-6. Leukemia 13: 327–334.
Zdzisinska B, Bojarska-Junak A, Dmoszynska A, Kandefer-Szerszen M . (2008). Abnormal cytokine production by bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells. Arch Immunol Ther Exp 56: 207–221.
Acknowledgements
We are indebted to Mrs Danielle Pineau for excellent technical help and to colleagues of the Clinical Hematology Departments of the hospitals of Nantes, La Roche-sur-Yon and Lorient for providing patient samples. The study was supported by grants from the Ligue Nationale contre le Cancer (Comité de Loire-Atlantique) and the Association pour la Recherche contre le Cancer. MB, CC and MV have been recipients of scholarships from the French Ministry of Research (MB: 2004–2007; CC: 2007–2010; MV: 2009–2010) and from the Association pour la Recherche contre le Cancer (MB, 2008). Authorship: MB, CC, MV, IC performed research, analysed data and helped to write the paper; MB and CC contributed equally. YJ analysed data and revised the paper. SH designed and performed research, analysed data and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Boissinot, M., Cleyrat, C., Vilaine, M. et al. Anti-inflammatory cytokines hepatocyte growth factor and interleukin-11 are over-expressed in Polycythemia vera and contribute to the growth of clonal erythroblasts independently of JAK2V617F. Oncogene 30, 990–1001 (2011). https://doi.org/10.1038/onc.2010.479
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.479