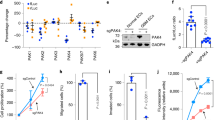
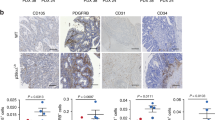
Abstract
Tumor cell plasticity enables certain types of highly malignant tumor cells to dedifferentiate and engage a plastic multipotent embryonic-like phenotype, which enables them to ‘adapt’ during tumor progression and escape conventional therapeutic strategies. This plastic phenotype of aggressive cancer cells enables them to express endothelial cell-specific markers and form tube-like structures, a phenotype that has been linked to aggressive behavior and poor prognosis. We demonstrate here that the transforming growth factor (TGF)-β co-receptor endoglin, an endothelial cell marker, is expressed by tumor cells and its expression correlates with tumor cell plasticity in two types of human cancer, Ewing sarcoma and melanoma. Moreover, endoglin expression was significantly associated with worse survival of Ewing sarcoma patients. Endoglin knockdown in tumor cells interferes with tumor cell plasticity and reduces invasiveness and anchorage-independent growth in vitro. Ewing sarcoma and melanoma cells with reduced endoglin levels showed reduced tumor growth in vivo. Mechanistically, we provide evidence that endoglin, while interfering with TGF-β signaling, is required for efficient bone morphogenetic protein, integrin, focal adhesion kinase and phosphoinositide-3-kinase signaling in order to maintain tumor cell plasticity. The present study delineates an important role of endoglin in tumor cell plasticity and progression of aggressive tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Altomonte M, Montagner R, Fonsatti E, Colizzi F, Cattarossi I, Brasoveanu LI et al. (1996). Expression and structural features of endoglin (CD105), a transforming growth factor β1 and β3 binding protein, in human melanoma. Br J Cancer 74: 1586–1591.
Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M et al. (2006). The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell 11: 313–323.
Bernabeu C, Lopez-Novoa JM, Quintanilla M . (2009). The emerging role of TGF-β superfamily coreceptors in cancer. Biochim Biophys Acta 1792: 954–973.
David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S . (2007). Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109: 1953–1961.
Dome B, Raso E, Dobos J, Meszaros L, Varga N, Puskas LG et al. (2005). Parallel expression of αIIbβ3 and αvβ3 integrins in human melanoma cells upregulates bFGF expression and promotes their angiogenic phenotype. Int J Cancer 116: 27–35.
Guo B, Slevin M, Li C, Parameshwar S, Liu D, Kumar P et al. (2004). CD105 inhibits transforming growth factor-β-Smad3 signalling. Anticancer Res 24: 1337–1345.
Hallani SE, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A et al. (2010). A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain 133: 973–982.
Hawinkels LJ, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E et al. (2010). Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res 70: 4141–4150.
Hendrix MJ, Seftor EA, Hess AR, Seftor RE . (2003). Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 3: 411–421.
Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA et al. (2001). Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA 98: 8018–8023.
Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM . (2007). Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer 7: 246–255.
Hendrix MJ, Seftor RE, Seftor EA, Gruman LM, Lee LM, Nickoloff BJ et al. (2002). Transendothelial function of human metastatic melanoma cells: role of the microenvironment in cell-fate determination. Cancer Res 62: 665–668.
Hess AR, Postovit LM, Margaryan NV, Seftor EA, Schneider GB, Seftor RE et al. (2005). Focal adhesion kinase promotes the aggressive melanoma phenotype. Cancer Res 65: 9851–9860.
Hess AR, Seftor EA, Gardner LM, Carles-Kinch K, Schneider GB, Seftor RE et al. (2001). Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2). Cancer Res 61: 3250–3255.
Hess AR, Seftor EA, Seftor RE, Hendrix MJ . (2003). Phosphoinositide 3-kinase regulates membrane Type 1-matrix metalloproteinase (MMP) and MMP-2 activity during melanoma cell vasculogenic mimicry. Cancer Res 63: 4757–4762.
Hillen F, Kaijzel EL, Castermans K, oude Egbrink MG, Lowik CW, Griffioen AW . (2008). A transgenic Tie2-GFP athymic mouse model; a tool for vascular biology in xenograft tumors. Biochem Biophys Res Commun 368: 364–367.
Kadkol SS, Lin AY, Barak V, Kalickman I, Leach L, Valyi-Nagy K et al. (2006). Osteopontin expression and serum levels in metastatic uveal melanoma: a pilot study. Invest Ophthalmol Vis Sci 47: 802–806.
Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M et al. (2004). Endoglin promotes endothelial cell proliferation and TGF-β/ALK1 signal transduction. EMBO J 23: 4018–4028.
Liu Y, Jovanovic B, Pins M, Lee C, Bergan RC . (2002). Over expression of endoglin in human prostate cancer suppresses cell detachment, migration and invasion. Oncogene 21: 8272–8281.
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe′er J et al. (1999). Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 155: 739–752.
Medrano EE . (2003). Repression of TGF-β signaling by the oncogenic protein SKI in human melanomas: consequences for proliferation, survival, and metastasis. Oncogene 22: 3123–3129.
Mueller AJ, Bartsch DU, Folberg R, Mehaffey MG, Boldt HC, Meyer M et al. (1998). Imaging the microvasculature of choroidal melanomas with confocal indocyanine green scanning laser ophthalmoscopy. Arch Ophthalmol 116: 31–39.
Ottaviano L, Schaefer KL, Gajewski M, Huckenbeck W, Baldus S, Rogel U et al. (2010). Molecular characterization of commonly used cell lines for bone tumor research: a trans-European EuroBoNet effort. Genes Chromosomes Cancer 49: 40–51.
Oxmann D, Held-Feindt J, Stark AM, Hattermann K, Yoneda T, Mentlein R . (2008). Endoglin expression in metastatic breast cancer cells enhances their invasive phenotype. Oncogene 27: 3567–3575.
Perez-Gomez E, Villa-Morales M, Santos J, Fernandez-Piqueras J, Gamallo C, Dotor J et al. (2007). A role for endoglin as a suppressor of malignancy during mouse skin carcinogenesis. Cancer Res 67: 10268–10277.
Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK . (2007). Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene 26: 4158–4170.
Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK . (2005). Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res 65: 448–456.
Scavelli C, Nico B, Cirulli T, Ria R, Di PG, Mangieri D et al. (2008). Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene 27: 663–674.
Seftor EA, Meltzer PS, Kirschmann DA, Pe′er J, Maniotis AJ, Trent JM et al. (2002). Molecular determinants of human uveal melanoma invasion and metastasis. Clin Exp Metastasis 19: 233–246.
Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M . (1997). Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res 3: 1031–1044.
Sharma N, Seftor RE, Seftor EA, Gruman LM, Heidger Jr PM, Cohen MB et al. (2002). Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate 50: 189–201.
Shirakawa K, Wakasugi H, Heike Y, Watanabe I, Yamada S, Saito K et al. (2002). Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int J Cancer 99: 821–828.
Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE et al. (2001). Molecular determinants of ovarian cancer plasticity. Am J Pathol 158: 1279–1288.
Staege MS, Hutter C, Neumann I, Foja S, Hattenhorst UE, Hansen G et al. (2004). DNA microarrays reveal relationship of Ewing family tumors to both endothelial and fetal neural crest-derived cells and define novel targets. Cancer Res 64: 8213–8221.
Sun B, Zhang S, Zhang D, Gu Y, Zhang W, Zhao X . (2007). The influence of different microenvironments on melanoma invasiveness and microcirculation patterns: an animal experiment study in the mouse model. J Cancer Res Clin Oncol 133: 979–985.
Szuhai K, Ijszenga M, Tanke HJ, Rosenberg C, Hogendoorn PC . (2006). Molecular cytogenetic characterization of four previously established and two newly established Ewing sarcoma cell lines. Cancer Genet Cytogenet 166: 173–179.
ten Dijke P, Arthur HM . (2007). Extracellular control of TGFβ signalling in vascular development and disease. Nat Rev Mol Cell Biol 8: 857–869.
ten Dijke P, Goumans MJ, Pardali E . (2008). Endoglin in angiogenesis and vascular diseases. Angiogenesis 11: 79–89.
Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW et al. (2006). Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med 12: 925–932.
van der Schaft DW, Hillen F, Pauwels P, Kirschmann DA, Castermans K, Egbrink MG et al. (2005). Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res 65: 11520–11528.
van der Schaft DW, Seftor RE, Seftor EA, Hess AR, Gruman LM, Kirschmann DA et al. (2004). Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst 96: 1473–1477.
Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM et al. (2006). Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 12: 642–649.
Wong VC, Chan PL, Bernabeu C, Law S, Wang LD, Li JL et al. (2008). Identification of an invasion and tumor-suppressing gene, Endoglin (ENG), silenced by both epigenetic inactivation and allelic loss in esophageal squamous cell carcinoma. Int J Cancer 123: 2816–2823.
Zhou Y, Dai DL, Martinka M, Su M, Zhang Y, Campos EI et al. (2005). Osteopontin expression correlates with melanoma invasion. J Invest Dermatol 124: 1044–1052.
Acknowledgements
We thank the members of our research groups for help and suggestions during the course of this work. We are grateful to Margriet Ouwens for assistance with PI3K activity assays, Inge Briaire-de Bruijn for excellent technical assistance with the TMA histochemical analysis, Dagmar Berghuis for help with the patient's clinical characteristics, and David de Gorter for critical reading of the paper. This study was supported by grants from the Centre of Biomedical Genetics, Dutch Cancer Society (RUL 2005-3371), the Ludwig Institute for Cancer Research, and FP6 EC Integrated Projects: Angiotargeting (504743), EC STREP Tumor-Host-Genomics (518198) and EuroBoNeT (018814).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Pardali, E., van der Schaft, D., Wiercinska, E. et al. Critical role of endoglin in tumor cell plasticity of Ewing sarcoma and melanoma. Oncogene 30, 334–345 (2011). https://doi.org/10.1038/onc.2010.418
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.418
Keywords
This article is cited by
-
TrkC, a novel prognostic marker, induces and maintains cell survival and metastatic dissemination of Ewing sarcoma by inhibiting EWSR1-FLI1 degradation
Cell Death & Disease (2022)
-
Endoglin and TGF-β signaling in glioblastoma
Cell and Tissue Research (2021)
-
The Tumor Microenvironment of Pediatric Sarcoma: Mesenchymal Mechanisms Regulating Cell Migration and Metastasis
Current Oncology Reports (2019)
-
The Role of Hypoxia and Cancer Stem Cells in Renal Cell Carcinoma Pathogenesis
Stem Cell Reviews and Reports (2015)
-
Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts
Oncogene (2014)