Abstract
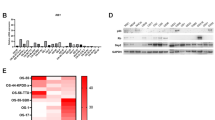
C-Src is infrequently mutated in human cancers but it mediates oncogenic signals of many activated growth factor receptors and thus remains a key target for cancer therapy. However, the broad function of Src in many cell types and processes requires evaluation of Src-targeted therapeutics within a normal developmental and immune-competent environment. In an effort to understand the appropriate clinical use of Src inhibitors, we tested an Src inhibitor, SKI-606 (bosutinib), in the MMTV-PyVmT transgenic mouse model of breast cancer. Tumor formation in this model is dependent on the presence of Src, but the necessity of Src kinase activity for tumor formation has not been determined. Furthermore, Src inhibitors have not been examined in an autochthonous tumor model that permits assessment of effects on different stages of tumor progression. Here we show that oral administration of SKI-606 inhibited the phosphorylation of Src in mammary tumors and caused a rapid decrease in the Ezh2 Polycomb group histone H3K27 methyltransferase and an increase in epithelial organization. SKI-606 prevented the appearance of palpable tumors in over 50% of the animals and stopped tumor growth in older animals with pre-existing tumors. These antitumor effects were accompanied by decreased cellular proliferation, altered tumor blood vessel organization and dramatically increased differentiation to lactational and epidermal cell fates. SKI-606 controls the development of mammary tumors by inducing differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D et al. (2006). Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439: 353–357.
Boschelli DH, Ye F, Wang YD, Dutia M, Johnson SL, Wu B et al. (2001). Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J Med Chem 44: 3965–3977.
Bracken AP, Helin K . (2009). Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer 9: 773–784.
Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA et al. (2008). Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 27: 7274–7284.
Cheung ATW, Young LJT, Chen PCY, Chao CY, Ndoye A, Barry PA et al. (1997). Microcirculation and metastasis in a new mouse mammary tumor model system. International J Oncol 11: 69–77.
Coluccia AM, Benati D, Dekhil H, De Filippo A, Lan C, Gambacorti-Passerini C . (2006). SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60(c-Src)-dependent tyrosine phosphorylation of beta-catenin and its nuclear signaling. Cancer Res 66: 2279–2286.
Courtneidge SA, Smith AE . (1983). Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature 303: 435–439.
Criscuoli ML, Nguyen M, Eliceiri BP . (2005). Tumor metastasis but not tumor growth is dependent on Src-mediated vascular permeability. Blood 105: 1508–1514.
Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA . (1999). Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 4: 915–924.
Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G et al. (2009). Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 136: 1122–1135.
Finn RS . (2008). Targeting Src in breast cancer. Ann Oncol 19: 1379–1386.
Galang CK, Muller WJ, Foos G, Oshima RG, Hauser CA . (2004). Changes in the expression of many Ets family transcription factors and of potential target genes in normal mammary tissue and tumors. J Biol Chem 279: 11281–11292.
Golas JM, Lucas J, Etienne C, Golas J, Discafani C, Sridharan L et al. (2005). SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res 65: 5358–5364.
Guy CT, Cardiff RD, Muller WJ . (1992). Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cellr Biol 12: 954–961.
Guy CT, Muthuswamy SK, Cardiff RD, Soriano P, Muller WJ . (1994). Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev 8: 23–32.
Ishizawar R, Parsons SJ . (2004). c-Src and cooperating partners in human cancer. Cancer Cell 6: 209–214.
Ishizawar RC, Miyake T, Parsons SJ . (2007). c-Src modulates ErbB2 and ErbB3 heterocomplex formation and function. Oncogene 26: 3503–3510.
Jain RK . (2005). Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307: 58–62.
Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, Rabbani SA . (2007). A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res 67: 1580–1588.
Kim H, Chan R, Dankort DL, Zuo D, Najoukas M, Park M et al. (2005a). The c-Src tyrosine kinase associates with the catalytic domain of ErbB-2: implications for ErbB-2 mediated signaling and transformation. Oncogene 24: 7599–7607.
Kim H, Laing M, Muller W . (2005b). c-Src-null mice exhibit defects in normal mammary gland development and ERalpha signaling. Oncogene 24: 5629–5636.
Kim LC, Song L, Haura EB . (2009). Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol 6: 587–595.
Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA et al. (2003). EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 100: 11606–11611.
Klein A, Guhl E, Zollinger R, Tzeng YJ, Wessel R, Hummel M et al. (2005). Gene expression profiling: cell cycle deregulation and aneuploidy do not cause breast cancer formation in WAP-SVT/t transgenic animals. J Mol Med 83: 362–376.
Kmiecik TE, Shalloway D . (1987). Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell 49: 65–73.
Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ et al. (2008). GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 13: 141–152.
Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM et al. (2006). Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125: 301–313.
Li X, Gonzalez ME, Toy K, Filzen T, Merajver SD, Kleer CG . (2009). Targeted overexpression of EZH2 in the mammary gland disrupts ductal morphogenesis and causes epithelial hyperplasia. Am J Pathol 175: 1246–1254.
Li Z, Tognon CE, Godinho FJ, Yasaitis L, Hock H, Herschkowitz JI et al. (2007). ETV6-NTRK3 fusion oncogene initiates breast cancer from committed mammary progenitors via activation of AP1 complex. Cancer Cell 12: 542–558.
Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ et al. (2003). Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 163: 2113–2126.
Liu M, Howes A, Lesperance J, Stallcup WB, Hauser CA, Kadoya K et al. (2005). Anti-tumor activity of rapamycin in a transgenic mouse model of ErbB2-dependent human breast cancer. Cancer Res 65: 5324–5336.
Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO et al. (2001). Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res 61: 8298–8305.
Man AK, Young LJT, Tynan J, Lesperance J, Egeblad M, Hauser CA et al. (2003). Ets2-dependent stromal regulation of mouse mammary tumors. Mol Cell Biol 23: 8614–8625.
Miyoshi K, Rosner A, Nozawa M, Byrd C, Morgan F, Landesman-Bollag E et al. (2002). Activation of different Wnt/beta-catenin signaling components in mammary epithelium induces transdifferentiation and the formation of pilar tumors. Oncogene 21: 5548–5556.
Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP . (1995). Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 375: 577–581.
Muthuswamy SK, Siegel PM, Dankort DL, Webster MA, Muller WJ . (1994). Mammary tumors expressing the neu proto-oncogene possess elevated c-Src tyrosine kinase activity. MolCell Biol 14: 735–743.
Neznanov N, Man AK, Yamamoto H, Hauser CA, Cardiff RD, Oshima RG . (1999). A single targeted Ets2 allele restricts development of mammary tumors in transgenic mice. Cancer Res 59: 4242–4246.
Oshima RG, Lesperance J, Munoz V, Hebbard L, Ranscht B, Sharan N et al. (2004). Angiogenic Acceleration of Neu Induced Mammary Tumor Progression and Metastasis. Cancer Res 64: 169–179.
Remsing Rix LL, Rix U, Colinge J, Hantschel O, Bennett KL, Stranzl T et al. (2009). Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia 23: 477–485.
Rosner A, Miyoshi K, Landesman-Bollag E, Xu X, Seldin DC, Moser AR et al. (2002). Pathway pathology: histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am J Pathol 161: 1087–1097.
Rudolph MC, McManaman JL, Phang T, Russell T, Kominsky DJ, Serkova NJ et al. (2007). Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol Genomics 28: 323–336.
Schwartzberg PL, Xing L, Hoffmann O, Lowell CA, Garrett L, Boyce BF et al. (1997). Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev 11: 2835–2844.
Silva CM, Shupnik MA . (2007). Integration of steroid and growth factor pathways in breast cancer: focus on signal transducers and activators of transcription and their potential role in resistance. Mol Endocrinol 21: 1499–1512.
Spector DH, Varmus HE, Bishop JM . (1978). Nucleotide sequences related to the transforming gene of avian sarcoma virus are present in DNA of uninfected vertebrates. Proc Natl Acad Sci USA 75: 4102–4106.
Summy JM, Gallick GE . (2006). Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res 12: 1398–1401.
Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B et al. (2008). Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 322: 1695–1699.
Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F et al. (2008). SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther 7: 1185–1194.
Watkin H, Richert MM, Lewis A, Terrell K, McManaman JP, Anderson SM . (2008). Lactation failure in Src knockout mice is due to impaired secretory activation. BMC Dev Biol 8: 6.
Webster MA, Cardiff RD, Muller WJ . (1995). Induction of mammary epithelial hyperplasias and mammary tumors in transgenic mice expressing a murine mammary tumor virus/activated c-src fusion gene. Proc Natl Acad Sci USA 92: 7849–7853.
Webster MA, Hutchinson JN, Rauh MJ, Muthuswamy SK, Anton M, Tortorice CG et al. (1998). Requirement for both Shc and phosphatidylinositol 3’ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol 18: 2344–2359.
Weis S, Cui J, Barnes L, Cheresh D . (2004). Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol 167: 223–229.
Acknowledgements
We thank Dr Mina Bissell, Lawrence Berkley National Laboratory for the gift of casein antibody, and Robert Abraham, Pfizer Research for his interest and support of the project. This work was supported by a collaborative research grant from Pfizer Research, and shared resource support from the NCI Cancer Center Support Grant 2 P30 CA030199 and R01 CA125255 (MK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Kim Arndt, Frank Boschelli, Lei Chen, Susan Q DeJoy, Veronica Diesl, Maxmillian Follettie, Jonathan Golas, Robert Rollins and Edward Rosfjord are employees of Pfizer (formerly Wyeth Research), which is pursuing clinical development of bosutinib. All the remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hebbard, L., Cecena, G., Golas, J. et al. Control of mammary tumor differentiation by SKI-606 (bosutinib). Oncogene 30, 301–312 (2011). https://doi.org/10.1038/onc.2010.412
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.412
Keywords
This article is cited by
-
SRC kinase-mediated signaling pathways and targeted therapies in breast cancer
Breast Cancer Research (2022)
-
Developmental Stage-Specific Embryonic Induction of HepG2 Cell Differentiation
Digestive Diseases and Sciences (2016)
-
Modulation of tumor fatty acids, through overexpression or loss of thyroid hormone responsive protein spot 14 is associated with altered growth and metastasis
Breast Cancer Research (2014)
-
Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross-talk
Nature Communications (2014)
-
miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT
Oncogene (2014)