Abstract
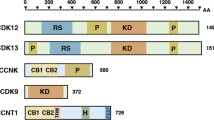
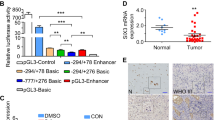
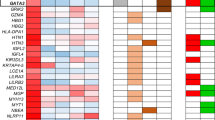
Almost all neuroblastoma tumors express excess levels of Cyclin D1 (CCND1) compared to normal tissues and other tumor types. Only a small percentage of these neuroblastoma tumors have high-level amplification of the Cyclin D1 gene. The other neuroblastoma tumors have equally high Cyclin D1 expression without amplification. Silencing of Cyclin D1 expression was previously found to trigger differentiation of neuroblastoma cells. Overexpression of Cyclin D1 is therefore one of the most frequent mechanisms with a postulated function in neuroblastoma pathogenesis. The cause for the Cyclin D1 overexpression is unknown. Here we show that Cyclin D1 overexpression results from transcriptional upregulation. To identify upstream regulators, we searched in mRNA profiles of neuroblastoma tumor series for transcription factors with expression patterns correlating to Cyclin D1. GATA3 most consistently correlated to Cyclin D1 in four independent data sets. We identified a highly conserved GATA3 binding site 27 bp upstream of the Cyclin D1 transcriptional start. Chromatin immune precipitation confirmed binding of GATA3 to the Cyclin D1 promoter. Overexpression of GATA3 induced Cyclin D1 promoter activity, which decreased after site-directed mutagenesis of the GATA3 binding site in the Cyclin D1 promoter. Silencing of GATA3 resulted in reduced Cyclin D1 promoter activity and reduced Cyclin D1 mRNA and protein levels. Moreover, GATA3 silencing caused differentiation that was similar to that caused by Cyclin D1 inhibition. These finding implicate GATA3 in Cyclin D1 overexpression in neuroblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A et al. (1995). Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem 270: 23589–23597.
Caren H, Erichsen J, Olsson L, Enerback C, Sjoberg RM, Abrahamsson J et al. (2008). High-resolution array copy number analyses for detection of deletion, gain, amplification and copy-neutral LOH in primary neuroblastoma tumors: four cases of homozygous deletions of the CDKN2A gene. BMC Genomics 9: 353.
Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M . (2007). Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer Res 67: 6477–6483.
Hanahan D, Weinberg RA . (2000). The hallmarks of cancer. Cell 100: 57–70.
Hoch RV, Thompson DA, Baker RJ, Weigel RJ . (1999). GATA-3 is expressed in association with estrogen receptor in breast cancer. Int J Cancer 84: 122–128.
Hong SJ, Choi HJ, Hong S, Huh Y, Chae H, Kim KS . (2008). Transcription factor GATA-3 regulates the transcriptional activity of dopamine beta-hydroxylase by interacting with Sp1 and AP4. Neurochem Res 33: 1821–1831.
Hong SJ, Huh Y, Chae H, Hong S, Lardaro T, Kim KS . (2006). GATA-3 regulates the transcriptional activity of tyrosine hydroxylase by interacting with CREB. J Neurochem 98: 773–781.
Kouros-Mehr H, Kim JW, Bechis SK, Werb Z . (2008). GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol 20: 164–170.
Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z . (2006). GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127: 1041–1055.
Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD . (2000). Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet 25: 209–212.
Maris JM, Hogarty MD, Bagatell R, Cohn SL . (2007). Neuroblastoma. Lancet 369: 2106–2120.
Michels E, Vandesompele J, De Preter K, Hoebeeck J, Vermeulen J, Schramm A et al. (2007). ArrayCGH-based classification of neuroblastoma into genomic subgroups. Genes Chromosomes Cancer 46: 1098–1108.
Molenaar JJ, Ebus ME, Koster J, van Sluis P, van Noesel CJ, Versteeg R et al. (2008). Cyclin D1 and CDK4 activity contribute to the undifferentiated phenotype in neuroblastoma. Cancer Res 68: 2599–2609.
Molenaar JJ, van Sluis P, Boon K, Versteeg R, Caron HN . (2003). Rearrangements and increased expression of cyclin D1 (CCND1) in neuroblastoma. Genes Chromosomes Cancer 36: 242–249.
Pei XH, Bai F, Smith MD, Usary J, Fan C, Pai SY et al. (2009). CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell 15: 389–401.
Sherr CJ . (1996). Cancer cell cycles. Science 274: 1672–1677.
Tsarovina K, Pattyn A, Stubbusch J, Muller F, van der WJ, Schneider C et al. (2004). Essential role of Gata transcription factors in sympathetic neuron development. Development 131: 4775–4786.
Usary J, Llaca V, Karaca G, Presswala S, Karaca M, He X et al. (2004). Mutation of GATA3 in human breast tumors. Oncogene 23: 7669–7678.
Vallania F, Schiavone D, Dewilde S, Pupo E, Garbay S, Calogero R et al. (2009). Genome-wide discovery of functional transcription factor binding sites by comparative genomics: the case of Stat3. Proc Natl Acad Sci USA 106: 5117–5122.
van Limpt V, Schramm A, van Lakeman A, Sluis P, Chan A, van Noesel M et al. (2004). The Phox2B homeobox gene is mutated in sporadic neuroblastomas. Oncogene 23: 9280–9288.
van Noesel MM, Versteeg R . (2004). Pediatric neuroblastomas: genetic and epigenetic ‘danse macabre’. Gene 325: 1–15.
van Roy N, Forus A, Myklebost O, Cheng NC, Versteeg R, Speleman F . (1995). Identification of two distinct chromosome 12-derived amplification units in neuroblastoma cell line NGP. Cancer Genet Cytogenet 82: 151–154.
Wilson BJ, Giguere V . (2008). Meta-analysis of human cancer microarrays reveals GATA3 is integral to the estrogen receptor alpha pathway. Mol Cancer 7: 49.
Acknowledgements
We kindly thank Dr RG Pestell for the full-length Cyclin D1 pA3 promoter construct that we used to generate our pGL3 Cyclin D1 promoter construct. The work was supported by Stichting Koningin Wilhelmina Fonds (KWF), Stichting Kindergeneeskundig Kankeronderzoek (SKK) and Kinderen Kankervrij (KiKa).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Molenaar, J., Ebus, M., Koster, J. et al. Cyclin D1 is a direct transcriptional target of GATA3 in neuroblastoma tumor cells. Oncogene 29, 2739–2745 (2010). https://doi.org/10.1038/onc.2010.21
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.21
Keywords
This article is cited by
-
Detection and identification of cis-regulatory elements using change-point and classification algorithms
BMC Genomics (2022)
-
Epigenetic deregulation of GATA3 in neuroblastoma is associated with increased GATA3 protein expression and with poor outcomes
Scientific Reports (2019)
-
Selective gene dependencies in MYCN-amplified neuroblastoma include the core transcriptional regulatory circuitry
Nature Genetics (2018)
-
Inhibition of CDK4/6 as a novel therapeutic option for neuroblastoma
Cancer Cell International (2015)
-
Progesterone receptor activation downregulates GATA3 by transcriptional repression and increased protein turnover promoting breast tumor growth
Breast Cancer Research (2014)