Abstract
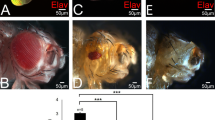
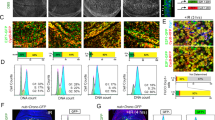
The tumor suppressor protein p53 has a critical role in safeguarding the integrity of the genome. Its functions are well understood but factors responsible for the transcriptional regulation of the p53 gene are almost entirely unknown. The DNA replication-related element (DRE)/DNA replication-related element-binding factor (DREF) transcriptional regulatory system is established as a master key to cell proliferation in Drosophila. DREF binds specifically to DRE sequences in the Drosophila p53 (dmp53) gene promoter as shown using anti-DREF antibodies in chromatin immunoprecipitation assays. Furthermore, a rough eye phenotype because of overexpression of DREF in Drosophila eye imaginal disks could be suppressed by half dose reduction of the dmp53 gene. In addition, the level of mRNA of dmp53 was decreased in DREF-knockdown cells and transient expression of the luciferase gene under control of the wild-type dmp53 gene promoter showed strong promoter activity in S2 cells, but this was almost completely abrogated with a DRE-mutated promoter. Requirement of DREs for dmp53 promoter activity was further confirmed by anti-β-galactosidase antibody-staining of various tissues from transgenic flies carrying dmp53 promoter-lacZ fusion genes. These results indicate that DREF is necessary for dmp53 gene promoter activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bauer JH, Poon PC, Glatt-Deeley H, Abrams JM, Helfand SL . (2005). Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends lifespan. Curr Biol 15: 2063–2068.
Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM . (2000). Drosophila p53 binds a damage response element at the reaper locus. Cell 101: 103–113.
Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG et al. (2004). Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol 24: 1219–1231.
Burns TF, El-Deiry WS . (1999). The p53 pathway and apoptosis. J Cell Physiol 181: 231–239.
Choi T, Cho N, Oh Y, Yoo M, Matsukage A, Ryu Y et al. (2000). The DNA replication-related element (DRE)-DRE-binding factor (DREF) system may be involved in the expression of the Drosophila melanogaster TBP gene. FEBS Lett 483: 71–77.
Galindo K, Smith PD . (2001). A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory. Genetics 159: 1059–1072.
Harris CC . (1996). Structure and function of the p53 tumor suppressor gene: clues for rational cancer therapeutic strategies. J Natl Cancer Inst 88: 1442–1455.
Hayashi Y, Kato M, Seto H, Yamaguchi M . (2006). Drosophila distal-less negatively regulates dDREF by inhibiting its DNA binding activity. Biochim Biophys Acta 1759: 359–366.
Hirose F, Ohshima N, Shiraki M, Inoue YH, Taguchi O, Nishi Y et al. (2001). Ectopic expression of DREF induces DNA synthesis, apoptosis, and unusual morphogenesis in the Drosophila eye imaginal disc: possible interaction with Polycomb and trithorax group proteins. Mol Cell Biol 21: 7231–7242.
Hirose F, Yamaguchi M, Handa H, Inomata Y, Matsukage A . (1993). Novel 8-base pair sequence (Drosophila DNA replication-related element) and specific binding factor involved in the expression of Drosophila genes for DNA polymerase α and proliferating cell nuclear antigen. J Biol Chem 268: 2092–2099.
Hirose F, Yamaguchi M, Kuroda K, Omori A, Hachiya T, Ikeda M et al. (1996). Isolation and characterization of cDNA for DREF, a promoter-activating factor for Drosophila DNA replication-related genes. J Biol Chem 271: 3930–3937.
Hirose F, Yamaguchi M, Matsukage A . (1999). Targeted expression of the DNA binding domain of DRE-binding factor, a Drosophila transcription factor, attenuates DNA replication of the salivary gland and eye imaginal disc. Mol Cell Biol 19: 6020–6028.
Hyun J, Jasper H, Bohmann D . (2005). DREF is required for efficient growth and cell cycle progression in Drosophila imaginal discs. Mol Cell Biol 25: 5590–5598.
Ida H, Suzusho N, Suyari O, Yoshida H, Ohno K, Hirose F et al. (2009). Genetic screening for modifiers of the DREF pathway in Drosophila melanogaster: identification and characterization of HP6 as a novel target of DREF. Nucleic Acids Res 37: 1423–1437.
Ida H, Yoshida H, Nakamura K, Yamaguchi M . (2007). Identification of the Drosophila eIF4A gene as a target of the DREF transcription factor. Exp Cell Res 313: 4208–4220.
Jasper H, Benes V, Atzberger A, Sauer S, Ansorge W, Bohmann D . (2002). A genomic switch at the transition from cell proliferation to terminal differentiation in the Drosophila eye. Dev Cell 3: 511–521.
Jin S, Martinek S, Joo WS, Wortman JR, Mirkovic N, Sali A et al. (2000). Identification and characterization of a p53 homologue in Drosophila melanogaster. Proc Natl Acad Sci 97: 7301–7306.
Zhao K, Hart CM, Laemmli UK . (1995). Visualization of chromosomal domains with boundary element-associated factor BEAF-32. Cell 81: 879–889.
Kim YS, Shin MJ, Yang DJ, Yamaguchi M, Park SY, Yoo MA . (2007). Transcriptional regulation of the Drosophila ANT gene by the DRE/DREF system. Genes Cells 12: 569–579.
Matsukage A, Hirose F, Yoo MA, Yamaguchi M . (2008). The DRE/DREF transcriptional regulatory system: a master key for cell proliferation. Biochim Biophys Acta 1779: 81–89.
Morrison TB, Weis JJ, Wittwer CT . (1998). Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques 24: 954–958 960, 962.
Nakamura K, Ida H, Yamaguchi M . (2008). Transcriptional regulation of the Drosophila moira and osa genes by the DREF pathway. Nucleic Acids Res 36: 3905–3915.
Ohno K, Hirose F, Sakaguchi K, Nishida Y, Matsukage A . (1996). Transcriptional regulation of the Drosophila CycA gene by the DNA replication-related element (DRE) and DRE binding factor (DREF). Nucleic Acids Res 24: 3942–3946.
Ohshima N, Takahashi M, Hirose F . (2003). Identification of a human homologue of the DREF transcription factor with a potential role in regulation of the histone H1 gene. J Biol Chem 278: 22928–22938.
Okudaira K, Ohno K, Yoshida H, Asano M, Hirose F, Yamaguchi M . (2005). Transcriptional regulation of the Drosophila orc2 gene by the DREF pathway. Biochim Biophys Acta 1732: 23–30.
Ollmann M, Young LM, Di Como CJ, Karim F, Belvin M, Robertson S et al. (2000). Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell 101: 91–101.
Rebollar E, Valadez-Graham V, Vazquez M, Reynaud E, Zurita M . (2006). Role of the p53 homologue from Drosophila melanogaster in the maintenance of histone H3 acetylation and response to UV-light irradiation. FEBS Lett 580: 642–648.
Robertson HM, Preston CR, Philips RW, Johnson-Schlitz DM, Benz WK, Engels WR . (1988). A stable genomic source of P-element tranposase in Drosophila melanogaster. Genetics 118: 461–470.
Ryu JR, Choi TY, Kwon EJ, Lee WH, Nishida Y, Hayashi Y et al. (1997). Transcriptional regulation of the Drosophila-raf proto-oncogene by the DNA replication-related element (DRE)/DRE-binding factor (DREF) system. Nucleic Acids Res 25: 794–799.
Sawado T, Hirose F, Takahashi Y, Sasaki T, Shinomiya T, Sakaguchi K et al. (1998). The DNA replication-related element (DRE)/DRE-binding factor system is a transcriptional regulator of the Drosophila E2F gene. J Biol Chem 273: 26042–26051.
Seto H, Hayashi Y, Kwon E, Taguchi O, Yamaguchi M . (2006). Antagonistic regulation of the Drosophila PCNA gene promoter by DREF and Cut. Genes Cells 11: 499–512.
Sharpless NE, Alson S, Chan S, Silver DP, Castrillon DH, DePinho RA . (2002). p16INK4a and p53 deficiency cooperate in tumorigenesis1. Cancer Res 62: 2761–2765.
Sogame N, Kim M, Abrams JM . (2003). Drosophila p53 preserves genomic stability by regulating cell death. Proc Natl Acad Sci USA 100: 4696–4701.
Somasundaram K . (2000). Tumor suppressor p53: regulation and function. Front Biosci 5: D424–D437.
Spradling AC . (1986). P-element-mediated transformation. In: Roberts DB (ed) Drosophila: A Practical Approach. IRL Press: Oxford.
Suyari O, Ida H, Yoshioka Y, Kato Y, Hashimoto R, Yamaguchi M . (2009). Identification of the Drosophila Mes4 gene as a novel target of the transcription factor DREF. Exp Cell Res 315: 1403–1414.
Takahashi Y, Hirose F, Matsukage A, Yamaguchi M . (1999). Identification of three conserved regions in the DREF transcription factors from Drosophila melanogaster and Drosophila virilis. Nucleic Acids Res 27: 510–516.
Takahashi Y, Yamaguchi M, Hirose F, Cotterill S, Kobayashi J, Miyajima S et al. (1996). DNA replication-related elements cooperate to enhance promoter activity of the Drosophila DNA polymerase α 73-kDa subunit gene. J Biol Chem 271: 14541–14547.
Thao DTP, Ida H, Yoshida H, Yamaguchi M . (2006). Identification of the Drosophila skpA gene as a novel target of the transcription factor DREF. Exp Cell Res 312: 3641–3650.
Tsuchiya A, Inoue YH, Ida H, Kawase Y, Okudaira K, Ohno K et al. (2007). Transcriptional regulation of the Drosophila rfc1 gene by the DRE-DREF pathway. Febs J 274: 1818–1832.
Yamaguchi M, Hayashi Y, Nishimoto Y, Hirose F, Matsukage A . (1995). A nucleotide sequence essential for the function of DRE, a common promoter element for Drosophila DNA replication-related genes. J Biol Chem 270: 15808–15814.
Yamaguchi M, Hirose F, Matsukage A . (1996). Roles of multiple promoter elements of the proliferating cell nuclear antigen gene during Drosophila development. Genes Cells 1: 47–58.
Yamashita D, Sano Y, Adachi Y, Okamoto Y, Osada H, Takahashi T et al. (2007). hDREF regulates cell proliferation and expression of ribosomal protein genes. Mol Cell Biol 27: 2003–2013.
Yamguchi M, Fumiko H, Yoshihiro HI, Michina S, Yuko H, Yoshimi N et al. (1999). Ectopic expression of human p53 inhibits entry into S phase and induces apoptosis in the Drosophila eye imaginal disc. Oncogen 18: 6767–6775.
Yoshida H, Kwon E, Hirose F, Otsuki K, Yamada M, Yamaguchi M . (2004). DREF is required for EGFR signalling during Drosophila wing vein development. Genes Cells 9: 935–944.
Acknowledgements
We are grateful to Dr M Abrams for dmp53 mutant stock, Dr Dean P Smith for kindly providing the casper-nls-LacZ plasmid, Dr Fumiko Hirose for the anti-DREF monoclonal antibodies and Dr Malcolm Moore for comments on the English language in the paper. This study was partially supported by a scholarship and grants from the KIT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Trong-Tue, N., Thao, D. & Yamaguchi, M. Role of DREF in transcriptional regulation of the Drosophila p53 gene. Oncogene 29, 2060–2069 (2010). https://doi.org/10.1038/onc.2009.483
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.483
Keywords
This article is cited by
-
The Drosophila histone methyltransferase NSD is positively regulated by the DRE/DREF system
Genes & Genomics (2018)
-
Phosphine inhibits transcription of the catalase gene through the DRE/DREF system in Drosophila melanogaster
Scientific Reports (2017)
-
The Hippo pathway as a target of the Drosophila DRE/DREF transcriptional regulatory pathway
Scientific Reports (2014)