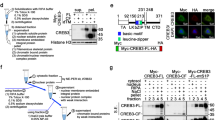
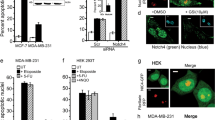
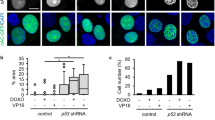
Abstract
DNA degradation is one of the biochemical hallmarks detected in apoptotic cells, and several nucleases have been reported to function cooperatively in this process. It has also been suggested that different sets of nucleases are activated by different stimuli, and induce distinct patterns of DNA degradation. Here we report that apoptosis-enhancing nuclease (AEN) is a novel direct target gene of p53. AEN is induced by p53 with various DNA damage, and its expression is regulated by the phosphorylation status of p53. We demonstrate that AEN is a typical exonuclease with conserved exonuclease domains Exo I–III, and it targets both single- and double-stranded DNA and RNA. AEN induces apoptosis by itself, and the conserved domains are essential for both AEN nuclease activity and its apoptosis-inducing ability. AEN possesses nuclear and nucleolar localization signals, and it translocates from the nucleolus to nucleoplasm upon apoptosis induction. We also show the dislocation of nucleophosmin in conjunction with the translocation of AEN to the nucleoplasm, indicating the ability of AEN in nucleolus disruption. In addition, AEN is shown to be required for efficient DNA fragmentation in p53-dependent apoptosis. These results suggest that AEN is an important downstream mediator of p53 in apoptosis induction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L et al. (1998). Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281: 1674–1677.
Blander G, Zalle N, Daniely Y, Taplick J, Gray MD, Oren M . (2002). DNA damage-induced translocation of the Werner helicase is regulated by acetylation. J Biol Chem 277: 50934–50940.
Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K et al. (1998). Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281: 1677–1679.
Chao C, Hergenhahn M, Kaeser MD, Wu Z, Saito S, Iggo R et al. (2003). Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J Biol Chem 278: 41028–41033.
Cordenonsi M, Montagner M, Adorno M, Zacchigna L, Martello G, Mamidi A et al. (2007). Integration of TGF-beta and Ras/MAPK signaling through p53 phosphorylation. Science 315: 840–843.
El-Deiry WS . (1998). Regulation of p53 downstream genes. Semin Cancer Biol 8: 345–357.
El-Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B . (1992). Definition of a consensus binding site for p53. Nat Genet 1: 45–49.
Hofseth LJ, Hussain SP, Harris CC . (2004). p53: 25 years after its discovery. Trends Pharmacol Sci 25: 177–181.
Houseley J, LaCava J, Tollervey D . (2006). RNA-quality control by the exosome. Nat Rev Mol Cell Biol 7: 529–539.
Jans DA, Xiao CY, Lam MH . (2000). Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays 22: 532–544.
Kaeser MD, Pebernard S, Iggo RD . (2004). Regulation of p53 stability and function in HCT116 colon cancer cells. J Biol Chem 279: 7598–7605.
Karni-Schmidt O, Friedler A, Zupnick A, McKinney K, Mattia M, Beckerman R et al. (2007). Energy-dependent nucleolar localization of p53 in vitro requires two discrete regions within the p53 carboxyl terminus. Oncogene 26: 3878–3891.
Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y et al. (2006). Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443: 998–1002.
Kurki S, Peltonen K, Laiho M . (2004). Nucleophosmin, HDM2 and p53: players in UV damage incited nucleolar stress response. Cell Cycle 3: 976–979.
Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN . (1998). Phosphorylation of p53 serine 15 increases interaction with CBP. J Biol Chem 273: 33048–33053.
Lee JH, Koh YA, Cho CK, Lee SJ, Lee YS, Bae S . (2005). Identification of a novel ionizing radiation-induced nuclease, AEN, and its functional characterization in apoptosis. Biochem Biophys Res Commun 337: 39–47.
Levine AJ . (1997). p53, the cellular gatekeeper for growth and division. Cell 88: 323–331.
Li LY, Luo X, Wang X . (2001). Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412: 95–99.
Liu G, Chen X . (2006). Regulation of the p53 transcriptional activity. J Cell Biochem 97: 448–458.
MacPherson D, Kim J, Kim T, Rhee BK, Van Oostrom CT, DiTullio RA et al. (2004). Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. EMBO J 23: 3689–3699.
Mayer C, Grummt I . (2005). Cellular stress and nucleolar function. Cell Cycle 4: 1036–1038.
Mayo LD, Seo YR, Jackson MW, Smith ML, Rivera Guzman J, Korgaonkar CK et al. (2005). Phosphorylation of human p53 at serine 46 determines promoter selection and whether apoptosis is attenuated or amplified. J Biol Chem 280: 25953–25959.
Moser MJ, Holley WR, Chatterjee A, Mian IS . (1997). The proofreading domain of Escherichia coli DNA polymerase I and other DNA and/or RNA exonuclease domains. Nucleic Acids Res 25: 5110–5118.
Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H . (2003). Degradation of chromosomal DNA during apoptosis. Cell Death Differ 10: 108–116.
Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T et al. (2000). Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288: 1053–1058.
Ohki R, Kawase T, Ohta T, Ichikawa H, Taya Y . (2007). Dissecting functional roles of p53 N-terminal transactivation domains by microarray expression analysis. Cancer Sci 98: 189–200.
Ohki R, Nemoto J, Murasawa H, Oda E, Inazawa J, Tanaka N et al. (2000). Reprimo, a new candidate mediator of the p53-mediated cell cycle arrest at the G2 phase. J Biol Chem 275: 22627–22630.
Parrish JZ, Xue D . (2006). Cuts can kill: the roles of apoptotic nucleases in cell death and animal development. Chromosoma 115: 89–97.
Prives C . (1998). Signaling to p53: breaking the MDM2-p53 circuit. Cell 95: 5–8.
Rowland RR, Yoo D . (2003). Nucleolar-cytoplasmic shuttling of PRRSV nucleocapsid protein: a simple case of molecular mimicry or the complex regulation by nuclear import, nucleolar localization and nuclear export signal sequences. Virus Res 95: 23–33.
Rubbi CP, Milner J . (2003). Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J 22: 6068–6077.
Saito S, Yamaguchi H, Higashimoto Y, Chao C, Xu Y, Fornace Jr AJ et al. (2003). Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J Biol Chem 278: 37536–37544.
Samejima K, Earnshaw WC . (2005). Trashing the genome: the role of nucleases during apoptosis. Nat Rev Mol Cell Biol 6: 677–688.
Schaetzlein S, Kodandaramireddy NR, Ju Z, Lechel A, Stepczynska A, Lilli DR et al. (2007). Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell 130: 863–877.
Shmueli A, Oren M . (2005). Life, death, and ubiquitin: taming the mule. Cell 121: 963–965.
Sun P, Yoshizuka N, New L, Moser BA, Li Y, Liao R et al. (2007). PRAK is essential for ras-induced senescence and tumor suppression. Cell 128: 295–308.
Tounekti O, Belehradek Jr J, Mir LM . (1995). Relationships between DNA fragmentation, chromatin condensation, and changes in flow cytometry profiles detected during apoptosis. Exp Cell Res 217: 506–516.
Unger T, Sionov RV, Moallem E, Yee CL, Howley PM, Oren M et al. (1999). Mutations in serines 15 and 20 of human p53 impair its apoptotic activity. Oncogene 18: 3205–3212.
Vogelstein B, Lane D, Levine AJ . (2000). Surfing the p53 network. Nature 408: 307–310.
Vousden KH, Lu X . (2002). Live or let die: the cell's response to p53. Nat Rev Cancer 2: 594–604.
Acknowledgements
This study was supported by the program for the promotion of Fundamental Studies in Health Sciences of the Pharmaceuticals and Medical Devices Agency (PMDA; to TO and HI), a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to YT), a Grant-in-Aid for Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labor and Welfare, Japan (to YT).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Kawase, T., Ichikawa, H., Ohta, T. et al. p53 target gene AEN is a nuclear exonuclease required for p53-dependent apoptosis. Oncogene 27, 3797–3810 (2008). https://doi.org/10.1038/onc.2008.32
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.32
Keywords
This article is cited by
-
Prevalence, causes and impact of TP53-loss phenocopying events in human tumors
BMC Biology (2023)
-
Systematic interrogation of mutation groupings reveals divergent downstream expression programs within key cancer genes
BMC Bioinformatics (2021)
-
Combined inhibition of XIAP and BCL2 drives maximal therapeutic efficacy in genetically diverse aggressive acute myeloid leukemia
Nature Cancer (2021)
-
Exploring the multiple roles of guardian of the genome: P53
Egyptian Journal of Medical Human Genetics (2020)
-
Plagioneurin B, a potent isolated compound induces apoptotic signalling pathways and cell cycle arrest in ovarian cancer cells
Apoptosis (2018)