Abstract
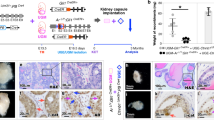
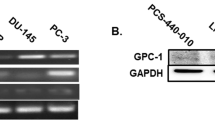
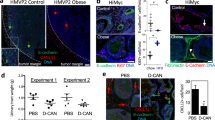
Transforming growth factor (TGF)-β is an important paracrine factor in tumorigenesis. Ligand binding of the type I and II TGF-β receptors initiate downstream signaling. The role of stromal TGF-β signaling in prostate cancer progression is unknown. In mice, the conditional stromal knockout of the TGF-β type II receptor expression (Tgfbr2fspKO) resulted in the development of prostatic intraepithelial neoplasia and progression to adenocarcinoma within 7 months. Clinically, we observed a loss of TGF-β receptor type II expression in 69% of human prostate cancer-associated stroma, compared to 15% of stroma associated with benign tissues (n=140, P-value <0.0001). To investigate the mechanism of paracrine TGF-β signaling in prostate cancer progression, we compared the effect of the prostatic stromal cells from Tgfbr2fspKO and floxed TGF-β type II receptor Tgfbr2floxE2/floxE2 mice on LNCaP human prostate cancer cells in vitro and tissue recombination xenografts. Induction of LNCaP cell proliferation and tumorigenesis was observed by Tgfbr2fspKO prostate stroma as a result of elevated Wnt3a expression. Neutralizing antibodies to Wnt3a reversed LNCaP tumorigenesis. The TGF-β inhibition of Wnt3a expression was in part through the suppression of Stat3 activity on the Wnt3a promoter. In conclusion, the frequent loss of stromal TGF-β type II receptor expression in human prostate cancer can relieve the paracrine suppression of Wnt3a expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- PIN:
-
prostatic intraepithelial neoplasia
- TβRII:
-
TGF-β type II receptor
- TGF-β:
-
transforming growth factor beta
- Tgfbr2floxE2/floxE2:
-
floxed TGF-β type II receptor
- Tgfbr2fspKO:
-
TGF-β type II receptor conditional fibroblast knockout
References
Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S et al. (2004a). TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303: 848–851.
Bhowmick NA, Moses HL . (2005). Tumor-stroma interactions. Curr Opin Genet Dev 15: 97–101.
Bhowmick NA, Neilson EG, Moses HL . (2004b). Stromal fibroblasts in cancer initiation and progression. Nature 432: 332–337.
Bierie B, Moses HL . (2006). TGF-beta and cancer. Cytokine Growth Factor Rev 17: 29–40.
Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL et al. (2007). Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest 117: 1305–1313.
Bright JJ, Sriram S . (1998). TGF-beta inhibits IL-12-induced activation of Jak-STAT pathway in T lymphocytes. J Immunol 161: 1772–1777.
Buck MB, Fritz P, Dippon J, Zugmaier G, Knabbe C . (2004). Prognostic significance of transforming growth factor beta receptor II in estrogen receptor-negative breast cancer patients. Clin Cancer Res 10: 491–498.
Calin GA, Gafa R, Tibiletti MG, Herlea V, Becheanu G, Cavazzini L et al. (2000). Genetic progression in microsatellite instability high (MSI-H) colon cancers correlates with clinico-pathological parameters: a study of the TGRbetaRII, BAX, hMSH3, hMSH6, IGFIIR and BLM genes. Int J Cancer 89: 230–235.
Cardillo MR, Petrangeli E, Perracchio L, Salvatori L, Ravenna L, Di Silverio F . (2000). Transforming growth factor-beta expression in prostate neoplasia. Anal Quant Cytol Histol 22: 1–10.
Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D et al. (2004). Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer 101: 1345–1356.
Chesire DR, Ewing CM, Gage WR, Isaacs WB . (2002). In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene 21: 2679–2694.
Clevers H . (2006). Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480.
Cunha GR . (1994). Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer 74: 1030–1044.
Davidson D, Bostwick DG, Qian J, Wollan PC, Oesterling JE, Rudders RA et al. (1995). Prostatic intraepithelial neoplasia is a risk factor for adenocarcinoma: predictive accuracy in needle biopsies. J Urol 154: 1295–1299.
Donjacour AA, Rosales A, Higgins SJ, Cunha GR . (1990). Characterization of antibodies to androgen-dependent secretory proteins of the mouse dorsolateral prostate. Endocrinology 126: 1343–1354.
Elliott RL, Blobe GC . (2005). Role of transforming growth factor Beta in human cancer. J Clin Oncol 23: 2078–2093.
Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R et al. (2003). Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4: 223–238.
Fujii H, Cunha GR, Norman JT . (1982). The induction of adenocarcinomatous differentiation in neoplastic bladder epithelium by an embryonic prostatic inductor. J Urol 128: 858–861.
Gerdes MJ, Larsen M, McBride L, Dang TD, Lu B, Rowley DR . (1998). Localization of transforming growth factor-beta1 and type II receptor in developing normal human prostate and carcinoma tissues. J Histochem Cytochem 46: 379–388.
Goggins M, Shekher M, Turnacioglu K, Yeo CJ, Hruban RH, Kern SE . (1998). Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res 58: 5329–5332.
Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD et al. (1999). Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res 59: 320–324.
Guo Y, Kyprianou N . (1999). Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res 59: 1366–1371.
Hayward SW, Del Buono R, Deshpande N, Hall PA . (1992). A functional model of adult human prostate epithelium. The role of androgens and stroma in architectural organisation and the maintenance of differentiated secretory function. J Cell Sci 102 (Part 2): 361–372.
Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD et al. (2001). Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 61: 8135–8142.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT et al. (1998). Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512.
Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG . (2007). Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res 13: 4769–4776.
Johnson ML, Rajamannan N . (2006). Diseases of Wnt signaling. Rev Endocr Metab Disord 7: 41–49.
Kim IY, Ahn HJ, Lang S, Oefelein MG, Oyasu R, Kozlowski JM et al. (1998). Loss of expression of transforming growth factor-beta receptors is associated with poor prognosis in prostate cancer patients. Clin Cancer Res 4: 1625–1630.
Kim IY, Ahn HJ, Zelner DJ, Shaw JW, Lang S, Kato M et al. (1996). Loss of expression of transforming growth factor beta type I and type II receptors correlates with tumor grade in human prostate cancer tissues. Clin Cancer Res 2: 1255–1261.
Kurita T, Medina RT, Mills AA, Cunha GR . (2004). Role of p63 and basal cells in the prostate. Development 131: 4955–4964.
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X et al. (2005). Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res 65: 5153–5162.
Kwok WK, Ling MT, Yuen HF, Wong YC, Wang X . (2007). Role of p14ARF in TWIST-mediated senescence in prostate epithelial cells. Carcinogenesis 28: 2467–2475.
Labbe E, Lock L, Letamendia A, Gorska AE, Gryfe R, Gallinger S et al. (2007). Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res 67: 75–84.
Morin PJ . (1999). beta-catenin signaling and cancer. Bioessays 21: 1021–1030.
Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H et al. (2000). Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann's organizer. Nature 403: 781–785.
Nissen RM, Yamamoto KR . (2000). The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev 14: 2314–2329.
Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A et al. (1991). A new nomenclature for int-1 and related genes: the Wnt gene family. Cell 64: 231.
Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H . (1984). Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature 307: 131–136.
Nusse R, Varmus HE . (1982). Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99–109.
Pasche B . (2001). Role of transforming growth factor beta in cancer. J Cell Physiol 186: 153–168.
Qian J, Bostwick DG, Takahashi S, Borell TJ, Herath JF, Lieber MM et al. (1995). Chromosomal anomalies in prostatic intraepithelial neoplasia and carcinoma detected by fluorescence in situ hybridization. Cancer Res 55: 5408–5414.
Qiao H, Hung W, Tremblay E, Wojcik J, Gui J, Ho J et al. (2002). Constitutive activation of met kinase in non-small-cell lung carcinomas correlates with anchorage-independent cell survival. J Cell Biochem 86: 665–677.
Roberts AB, Wakefield LM . (2003). The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci USA 100: 8621–8623.
Royuela M, De Miguel MP, Bethencourt FR, Sanchez-Chapado M, Fraile B, Paniagua R . (1998). Transforming growth factor beta 1 and its receptor types I and II. Comparison in human normal prostate, benign prostatic hyperplasia, and prostatic carcinoma. Growth Factors 16: 101–110.
Sam MR, Elliott BE, Mueller CR . (2007). A novel activating role of SRC and STAT3 on HGF transcription in human breast cancer cells. Mol Cancer 6: 69.
Schmid P, Itin P, Rufli T . (1995). In situ analysis of transforming growth factor-beta s (TGF-beta 1, TGF-beta 2, TGF-beta 3), and TGF-beta type II receptor expression in malignant melanoma. Carcinogenesis 16: 1499–1503.
Shi Y, Massague J . (2003). Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700.
Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S . (1998). Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 3: 659–670.
Turner T, Bern HA, Young P, Cunha GR . (1990). Serum-free culture of enriched mouse anterior and ventral prostatic epithelial cells in collagen gel. In Vitro Cell Dev Biol 26: 722–730.
Verras M, Sun Z . (2006). Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett 237: 22–32.
Voeller HJ, Truica CI, Gelmann EP . (1998). Beta-catenin mutations in human prostate cancer. Cancer Res 58: 2520–2523.
Wang Y, Hayward SW, Donjacour AA, Young P, Jacks T, Sage J et al. (2000). Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res 60: 6008–6017.
Wojcik EJ, Sharifpoor S, Miller NA, Wright TG, Watering R, Tremblay EA et al. (2006). A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. Oncogene 25: 2773–2784.
Wong YC, Cunha GR, Hayashi N . (1992). Effects of mesenchyme of the embryonic urogenital sinus and neonatal seminal vesicle on the cytodifferentiation of the Dunning tumor: ultrastructural study. Acta Anat (Basel) 143: 139–150.
Yardy GW, Brewster SF . (2005). Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis 8: 119–126.
Zhang Z, Xie D, Li X, Wong YC, Xin D, Guan XY et al. (2007). Significance of TWIST expression and its association with E-cadherin in bladder cancer. Hum Pathol 38: 598–606.
Acknowledgements
Dr Xiuping Yu (Vanderbilt University) generously provided primers for the Wnt isoforms. This study was supported by the DOD through DAMD (W81XWH-04-1-0046 to NAB) as well as through NIH Grants (CA108646, CA126505 to NAB and FGM079879A to VRP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Li, X., Placencio, V., Iturregui, J. et al. Prostate tumor progression is mediated by a paracrine TGF-β/Wnt3a signaling axis. Oncogene 27, 7118–7130 (2008). https://doi.org/10.1038/onc.2008.293
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.293
Keywords
This article is cited by
-
Extracellular vesicles from biological fluids as potential markers in castration resistant prostate cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Runt related transcription factor-1 plays a central role in vessel co-option of colorectal cancer liver metastases
Communications Biology (2021)
-
Prostate Cancer Immunotherapy—Finally in From the Cold?
Current Oncology Reports (2021)
-
The great escape: tumour cell plasticity in resistance to targeted therapy
Nature Reviews Drug Discovery (2020)
-
Enabling precision medicine by unravelling disease pathophysiology: quantifying signal transduction pathway activity across cell and tissue types
Scientific Reports (2019)