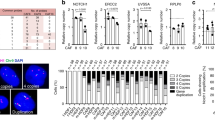
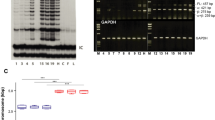
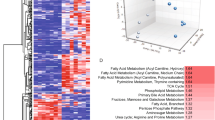
Abstract
Basal cell carcinoma of the skin is the most common type of cancer in humans. The majority of these tumors displays aberrant activation of the SONIC HEDGEHOG (SHH)/PATCHED pathway, triggered by mutations in the PATCHED tumor suppressor gene, which encodes a transmembrane receptor of SHH. In this study, we took advantage of the natural genotype (PATCHED+/−) of healthy keratinocytes expanded from patients with the nevoid basal cell carcinoma or Gorlin syndrome to mimic heterozygous somatic mutations thought to occur in the PATCHED gene early upon basal cell carcinoma development in the general population. PATCHED+/− epidermis developed on a dermal equivalent containing wild-type (WT) PATCHED+/+ fibroblasts exhibited striking invasiveness and hyperproliferation, as well as marked differentiation impairment. Deciphering the phenotype of PATCHED+/− keratinocytes revealed slight increases of the transcriptional activators GLI1 and GLI2—the latter known to provoke basal cell carcinoma-like tumors when overexpressed in transgenic mice. PATCHED+/− keratinocytes also showed a substantial increase of the cell cycle regulator cyclin D1. These data show for the first time the physiological impact of constitutive heterozygous PATCHED mutations in primary human keratinocytes and strongly argue for a yet elusive mechanism of haploinsufficiency leading to cancer proneness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Adolphe C, Hetherington R, Ellis T, Wainwright B . (2006). Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res 66: 2081–2088.
Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP et al. (1999). Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med 5: 1285–1291.
Bernerd F, Asselineau D, Vioux C, Chevallier-Lagente O, Bouadjar B, Sarasin A et al. (2001). Clues to epidermal cancer proneness revealed by reconstruction of DNA repair-deficient xeroderma pigmentosum skin in vitro. Proc Natl Acad Sci USA 98: 7817–7822.
Bigelow RL, Jen EY, Delehedde M, Chari NS, McDonnell TJ . (2005). Sonic hedgehog induces epidermal growth factor dependent matrix infiltration in HaCaT keratinocytes. J Invest Dermatol 124: 457–465.
Boutet N, Bignon YJ, Drouin-Garraud V, Sarda P, Longy M, Lacombe D et al. (2003). Spectrum of PTCH1 mutations in French patients with Gorlin syndrome. J Invest Dermatol 121: 478–481.
Brellier F, Valin A, Chevallier-Lagente O, Gorry P, Avril MF, Magnaldo T . (2008). Ultraviolet responses of Gorlin syndrome primary skin cells. Br J Dermatol (28 May 2008, e-pub ahead of print).
Chidambaram A, Dean M . (1996). Genetics of the nevoid basal cell carcinoma syndrome. Adv Cancer Res 70: 49–61.
Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A . (1997). Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature 389: 876–881.
DePinho RA . (2000). The age of cancer. Nature 408: 248–254.
Evans T, Boonchai W, Shanley S, Smyth I, Gillies S, Georgas K et al. (2000). The spectrum of patched mutations in a collection of Australian basal cell carcinomas. Hum Mutat 16: 43–48.
Fan H, Khavari PA . (1999). Sonic hedgehog opposes epithelial cell cycle arrest. J Cell Biol 147: 71–76.
Gailani MR, Stahle-Backdahl M, Leffell DJ, Glynn M, Zaphiropoulos PG, Pressman C et al. (1996). The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet 14: 78–81.
Gorlin RJ . (1987). Nevoid basal-cell carcinoma syndrome. Medicine (Baltimore) 66: 98–113.
Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC et al. (2000). Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet 24: 216–217.
Hahn H, Christiansen J, Wicking C, Zaphiropoulos PG, Chidambaram A, Gerrard B et al. (1996). A mammalian patched homolog is expressed in target tissues of sonic hedgehog and maps to a region associated with developmental abnormalities. J Biol Chem 271: 12125–12128.
Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM et al. (1996). Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272: 1668–1671.
Kenney AM, Rowitch DH . (2000). Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol 20: 9055–9067.
Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ . (1996). Biochemical evidence that patched is the Hedgehog receptor. Nature 384: 176–179.
Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA et al. (2003). Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev 17: 282–294.
Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG et al. (2000). Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci USA 97: 3438–3443.
Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein Jr EH, Scott MP . (1997). Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276: 817–821.
Pruvost-Balland C, Gorry P, Boutet N, Magnaldo T, Mamelle G, Margulis A et al. (2006). [Clinical and genetic study in 22 patients with basal cell nevus syndrome]. Ann Dermatol Venereol 133: 117–123.
Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Ikram MS et al. (2004). The zinc-finger transcription factor GLI2 antagonizes contact inhibition and differentiation of human epidermal cells. Oncogene 23: 1263–1274.
Reifenberger J, Wolter M, Knobbe CB, Kohler B, Schonicke A, Scharwachter C et al. (2005). Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol 152: 43–51.
Rheinwald JG, Green H . (1975). Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6: 331–343.
Soufir N, Gerard B, Portela M, Brice A, Liboutet M, Saiag P et al. (2006). PTCH mutations and deletions in patients with typical nevoid basal cell carcinoma syndrome and in patients with a suspected genetic predisposition to basal cell carcinoma: a French study. Br J Cancer 95: 548–553.
Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL et al. (1996). The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384: 129–134.
Teh MT, Blaydon D, Chaplin T, Foot NJ, Skoulakis S, Raghavan M et al. (2005). Genomewide single nucleotide polymorphism microarray mapping in basal cell carcinomas unveils uniparental disomy as a key somatic event. Cancer Res 65: 8597–8603.
Unden AB, Zaphiropoulos PG, Bruce K, Toftgard R, Stahle-Backdahl M . (1997). Human patched (PTCH) mRNA is overexpressed consistently in tumor cells of both familial and sporadic basal cell carcinoma. Cancer Res 57: 2336–2340.
Acknowledgements
VB, AV, SB, have contributed equally to this work. FB and AV were recipients of PhD fellowships from the CNRS and the Ligue Nationale contre le Cancer, and MESR, respectively. SB was an IGR post-doctoral fellow. We gratefully thank Dr Françoise Bernerd, Dr Howard Green, Ms Valérie Vélasco, Virginie Marty, Dr Rune Toftgard, Dr Paule Opolon, and Marianne Brown-Luedi for their kind help. This work was supported by the CNRS, the ARC (no. 9500), the Fondation de l’Avenir, the SFD and the AFM.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Brellier, F., Bergoglio, V., Valin, A. et al. Heterozygous mutations in the tumor suppressor gene PATCHED provoke basal cell carcinoma-like features in human organotypic skin cultures. Oncogene 27, 6601–6606 (2008). https://doi.org/10.1038/onc.2008.260
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.260