Abstract
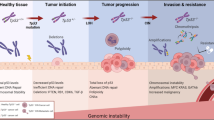
The phosphatase and tensin homolog deleted on chromosome 10 (PTEN) tumor suppressor is a phosphatase that antagonizes the phosphoinositol-3-kinase/AKT signaling pathway and suppresses cell survival as well as cell proliferation. PTEN is the second most frequently mutated gene in human cancer after p53. Germline mutations of PTEN have been found in cancer susceptibility syndromes, such as Cowden syndrome, in which over 80% of patients have mutations of PTEN. Homozygous deletion of Pten causes embryonic lethality, suggesting that PTEN is essential for embryonic development. Mice heterozygous for Pten develop spontaneous tumors in a variety of organs comparable with the spectrum of its mutations in human cancer. The mechanisms of PTEN functions in tumor suppression are currently under intense investigation. Recent studies demonstrate that PTEN plays an essential role in the maintenance of chromosomal stability and that loss of PTEN leads to massive alterations of chromosomes. The tumor suppressor p53 is known as a guardian of the genome that mediates the cellular response to environmental stress, leading to cell cycle arrest or cell death. Through completely different mechanisms, PTEN also protects the genome from instability. Thus, we propose that PTEN is a new guardian of the genome. In this review, we will discuss new discoveries on the role of PTEN in tumor suppression and explore mechanisms by which PTEN maintains genomic stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Abounader R, Reznik T, Colantuoni C, Martinez-Murillo F, Rosen EM, Laterra J . (2004). Regulation of c-Met-dependent gene expression by PTEN. Oncogene 23: 9173–9182.
Agrawal S, Pilarski R, Eng C . (2005). Different splicing defects lead to differential effects downstream of the lipid and protein phosphatase activities of PTEN. Hum Mol Genet 14: 2459–2468.
Backman SA, Ghazarian D, So K, Sanchez O, Wagner KU, Hennighausen L et al. (2004). Early onset of neoplasia in the prostate and skin of mice with tissue-specific deletion of Pten. Proc Natl Acad Sci USA 101: 1725–1730.
Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J et al. (2001). Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte–Duclos disease. Nat Genet 29: 396–403.
Bai F, Pei XH, Pandolfi PP, Xiong Y . (2006). p18 Ink4c and Pten constrain a positive regulatory loop between cell growth and cell cycle control. Mol Cell Biol 26: 4564–4576.
Baker SJ . (2007). PTEN enters the nuclear age. Cell 128: 25–28.
Bilbao C, Rodriguez G, Ramirez R, Falcon O, Leon L, Chirino R et al. (2006). The relationship between microsatellite instability and PTEN gene mutations in endometrial cancer. Int J Cancer 119: 563–570.
Bischoff FZ, Strong LC, Yim SO, Pratt DR, Siciliano MJ, Giovanella BC et al. (1991). Tumorigenic transformation of spontaneously immortalized fibroblasts from patients with a familial cancer syndrome. Oncogene 6: 183–186.
Borresen-Dale AL . (2003). TP53 and breast cancer. Hum Mutat 21: 292–300.
Cantley LC, Neel BG . (1999). New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 96: 4240–4245.
Chang CJ, Freeman DJ, Wu H . (2004). PTEN regulates Mdm2 expression through the P1 promoter. J Biol Chem 279: 29841–29848.
Chang CJ, Mulholland DJ, Valamehr B, Mosessian S, Sellers WR, Wu H . (2008). PTEN nuclear localization is regulated by oxidative stress and mediates p53-dependent tumor suppression. Mol Cell Biol 28: 3281–3289.
Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M et al. (2005). Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436: 725–730.
Chung JH, Ginn-Pease ME, Eng C . (2005). Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) has nuclear localization signal-like sequences for nuclear import mediated by major vault protein. Cancer Res 65: 4108–4116.
Cully M, You H, Levine AJ, Mak TW . (2006). Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 6: 184–192.
Das S, Dixon JE, Cho W . (2003). Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci USA 100: 7491–7496.
Di Cristofano A, Pandolfi PP . (2000). The multiple roles of PTEN in tumor suppression. Cell 100: 387–390.
Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP . (1998). Pten is essential for embryonic development and tumour suppression. Nat Genet 19: 348–355.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery Jr CA, Butel JS et al. (1992). Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356: 215–221.
Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS . (2008). PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a). Proc Natl Acad Sci USA 105: 2622–2627.
Eng C . (2003). PTEN: one gene, many syndromes. Hum Mutat 22: 183–198.
Eng C, Peacocke M . (1998). PTEN and inherited hamartoma-cancer syndromes. Nat Genet 19: 223.
Fanning AS, Anderson JM . (1999). Protein modules as organizers of membrane structure. Curr Opin Cell Biol 11: 432–439.
Flores-Delgado G, Liu CW, Sposto R, Berndt N . (2007). A limited screen for protein interactions reveals new roles for protein phosphatase 1 in cell cycle control and apoptosis. J Proteome Res 6: 1165–1175.
Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R et al. (2003). PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3: 117–130.
Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM et al. (1986). A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 323: 643–646.
Fujisawa H, Reis RM, Nakamura M, Colella S, Yonekawa Y, Kleihues P et al. (2000). Loss of heterozygosity on chromosome 10 is more extensive in primary (de novo) than in secondary glioblastomas. Lab Invest 80: 65–72.
Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H . (1999). The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA 96: 10182–10187.
Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H . (2000). Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res 60: 7033–7038.
Gildea JJ, Herlevsen M, Harding MA, Gulding KM, Moskaluk CA, Frierson HF et al. (2004). PTEN can inhibit in vitro organotypic and in vivo orthotopic invasion of human bladder cancer cells even in the absence of its lipid phosphatase activity. Oncogene 23: 6788–6797.
Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U et al. (2000). Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol 156: 1693–1700.
Ginn-Pease ME, Eng C . (2003). Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0-G1 in MCF-7 cells. Cancer Res 63: 282–286.
Gu J, Tamura M, Yamada KM . (1998). Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol 143: 1375–1383.
Guan KL, Jenkins CW, Li Y, Nichols MA, Wu X, O'Keefe CL et al. (1994). Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev 8: 2939–2952.
Hong TM, Yang PC, Peck K, Chen JJ, Yang SC, Chen YC et al. (2000). Profiling the downstream genes of tumor suppressor PTEN in lung cancer cells by complementary DNA microarray. Am J Respir Cell Mol Biol 23: 355–363.
Iida S, Nakamura Y, Fujii H, Kimura M, Moriwaki K . (1998). A heterozygous germline mutation of the PTEN/MMAC1 gene in a patient with Cowden disease. Int J Mol Med 1: 565–568.
Iida S, Ono A, Sayama K, Hamaguchi T, Fujii H, Nakajima H et al. (2000). Accelerated decline of blood glucose after intravenous glucose injection in a patient with Cowden disease having a heterozygous germline mutation of the PTEN/MMAC1 gene. Anticancer Res 20: 1901–1904.
Kanaseki T, Torigoe T, Hirohashi Y, Hirai I, Himi T, Sato N . (2002). Identification of germline mutation of PTEN gene and analysis of apoptosis resistance of the lymphocytes in a patient with Cowden disease. Pathobiology 70: 34–39.
Kang-Park S, Lee YI . (2003). PTEN modulates insulin-like growth factor II (IGF-II)-mediated signaling; the protein phosphatase activity of PTEN downregulates IGF-II expression in hepatoma cells. FEBS Lett 545: 203–208.
Kang-Park S, Lee YI, Lee YI . (2003). PTEN modulates insulin-like growth factor II (IGF-II)-mediated signaling; the protein phosphatase activity of PTEN downregulates IGF-II expression in hepatoma cells. FEBS Lett 545: 203–208.
Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV et al. (1992). A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71: 587–597.
Kato H, Kato S, Kumabe T, Sonoda Y, Yoshimoto T, Kato S et al. (2000). Functional evaluation of p53 and PTEN gene mutations in gliomas. Clin Cancer Res 6: 3937–3943.
Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N et al. (2003). Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development 130: 1691–1700.
Koul A, Willen R, Bendahl PO, Nilbert M, Borg A . (2002). Distinct sets of gene alterations in endometrial carcinoma implicate alternate modes of tumorigenesis. Cancer 94: 2369–2379.
Koul D, Shen R, Shishodia S, Takada Y, Bhat KP, Reddy SA et al. (2007). PTEN down regulates AP-1 and targets c-fos in human glioma cells via PI3-kinase/Akt pathway. Mol Cell Biochem 300: 77–87.
Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C . (2002). Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet 32: 355–357.
Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S et al. (2000). A role for nuclear PTEN in neuronal differentiation. J Neurosci 20: 1404–1413.
Lane DP . (1992). Cancer. p53, guardian of the genome. Nature 358: 15–16.
Langerod A, Zhao H, Borgan O, Nesland JM, Bukholm IR, Ikdahl T et al. (2007). TP53 mutation status and gene expression profiles are powerful prognostic markers of breast cancer. Breast Cancer Res 9: R30.
Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y et al. (1999). Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99: 323–334.
Leslie NR, Downes CP . (2004). PTEN function: how normal cells control it and tumour cells lose it. Biochem J 382: 1–11.
Leslie NR, Yang X, Downes CP, Weijer CJ . (2007). PtdIns(3,4,5)P(3)-dependent and -independent roles for PTEN in the control of cell migration. Curr Biol 17: 115–125.
Levine AJ . (1997). p53, the cellular gatekeeper for growth and division. Cell 88: 323–331.
Levine AJ, Finlay CA, Hinds PW . (2004). P53 is a tumor suppressor gene. Cell 116: S67–S69, 61 p following S69.
Li DM, Sun H . (1997). TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 57: 2124–2129.
Li DM, Sun H . (1998). PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA 95: 15406–15411.
Li J, Simpson L, Takahashi M, Miliaresis C, Myers MP, Tonks N et al. (1998). The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer Res 58: 5667–5672.
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI et al. (1997). PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275: 1943–1947.
Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z et al. (1997). Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 16: 64–67.
Liliental J, Moon SY, Lesche R, Mamillapalli R, Li D, Zheng Y et al. (2000). Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol 10: 401–404.
Liu F, Wagner S, Campbell RB, Nickerson JA, Schiffer CA, Ross AH . (2005a). PTEN enters the nucleus by diffusion. J Cell Biochem 96: 221–234.
Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK . (2005b). Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol 25: 6211–6224.
Livingstone LR, White A, Sprouse J, Livanos E, Jacks T, Tlsty TD . (1992). Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell 70: 923–935.
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T . (1993). p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362: 847–849.
Maehama T, Dixon JE . (1998). The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378.
Mahimainathan L, Choudhury GG . (2004). Inactivation of platelet-derived growth factor receptor by the tumor suppressor PTEN provides a novel mechanism of action of the phosphatase. J Biol Chem 279: 15258–15268.
Maier D, Jones G, Li X, Schonthal AH, Gratzl O, Van Meir EG et al. (1999). The PTEN lipid phosphatase domain is not required to inhibit invasion of glioma cells. Cancer Res 59: 5479–5482.
Malkin D, Li FP, Strong LC, Fraumeni Jr JF, Nelson CE, Kim DH et al. (1990). Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250: 1233–1238.
Matsushima-Nishiu M, Unoki M, Ono K, Tsunoda T, Minaguchi T, Kuramoto H et al. (2001). Growth and gene expression profile analyses of endometrial cancer cells expressing exogenous PTEN. Cancer Res 61: 3741–3749.
Mayo LD, Donner DB . (2002). The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci 27: 462–467.
Mayo MW, Madrid LV, Westerheide SD, Jones DR, Yuan XJ, Baldwin Jr AS et al. (2002). PTEN blocks tumor necrosis factor-induced NF-kappa B-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J Biol Chem 277: 11116–11125.
McGarrity TJ, Wagner Baker MJ, Ruggiero FM, Thiboutot DM, Hampel H, Zhou XP et al. (2003). GI polyposis and glycogenic acanthosis of the esophagus associated with PTEN mutation positive Cowden syndrome in the absence of cutaneous manifestations. Am J Gastroenterol 98: 1429–1434.
Medema RH, Herrera RE, Lam F, Weinberg RA . (1995). Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci USA 92: 6289–6293.
Minaguchi T, Waite KA, Eng C . (2006). Nuclear localization of PTEN is regulated by Ca(2+) through a tyrosil phosphorylation-independent conformational modification in major vault protein. Cancer Res 66: 11677–11682.
Moon SK, Kim HM, Kim CH . (2004). PTEN induces G1 cell cycle arrest and inhibits MMP-9 expression via the regulation of NF-kappaB and AP-1 in vascular smooth muscle cells. Arch Biochem Biophys 421: 267–276.
Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA et al. (1998). The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA 95: 13513–13518.
Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH et al. (1997). P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA 94: 9052–9057.
Nan B, Snabboon T, Unni E, Yuan XJ, Whang YE, Marcelli M . (2003). The PTEN tumor suppressor is a negative modulator of androgen receptor transcriptional activity. J Mol Endocrinol 31: 169–183.
Okumura K, Zhao M, DePinho RA, Furnari FB, Cavenee WK . (2005). PTEN: a novel anti-oncogenic function independent of phosphatase activity. Cell Cycle 4: 540–542.
Paramio JM, Navarro M, Segrelles C, Gomez-Casero E, Jorcano JL . (1999). PTEN tumour suppressor is linked to the cell cycle control through the retinoblastoma protein. Oncogene 18: 7462–7468.
Park MJ, Kim MS, Park IC, Kang HS, Yoo H, Park SH et al. (2002). PTEN suppresses hyaluronic acid-induced matrix metalloproteinase-9 expression in U87 MG glioblastoma cells through focal adhesion kinase dephosphorylation. Cancer Res 62: 6318–6322.
Parsons R . (2004). Human cancer, PTEN and the PI-3 kinase pathway. Semin Cell Dev Biol 15: 171–176.
Perren A, Komminoth P, Saremaslani P, Matter C, Feurer S, Lees JA et al. (2000). Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cells. Am J Pathol 157: 1097–1103.
Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM et al. (1999). Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA 96: 1563–1568.
Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L et al. (2005). Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell 7: 193–204.
Puc J, Parsons R . (2005). PTEN loss inhibits CHK1 to cause double stranded-DNA breaks in cells. Cell Cycle 4: 927–929.
Quelle DE, Ashmun RA, Hannon GJ, Rehberger PA, Trono D, Richter KH et al. (1995). Cloning and characterization of murine p16INK4a and p15INK4b genes. Oncogene 11: 635–645.
Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A . (2004). Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303: 1179–1181.
Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM et al. (1999). Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA 96: 2110–2115.
Saal LH, Gruvberger-Saal SK, Persson C, Lovgren K, Jumppanen M, Staaf J et al. (2008). Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet 40: 102–107.
Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML et al. (1999). Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis. Cancer Res 59: 1820–1824.
Sansal I, Sellers WR . (2004). The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol 22: 2954–2963.
Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP et al. (2007). Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128: 157–170.
Shin KH, Kim JM, Rho KS, Park KH, Oh JE, Min BM . (2002). Inactivation of the PTEN gene by mutation, exonic deletion, and loss of transcript in human oral squamous cell carcinomas. Int J Oncol 21: 997–1001.
Shmueli A, Oren M . (2004). Regulation of p53 by Mdm2: fate is in the numbers. Mol Cell 13: 4–5.
Shmueli A, Oren M . (2005). Life, death, and ubiquitin: taming the mule. Cell 121: 963–965.
Simpson L, Parsons R . (2001). PTEN: life as a tumor suppressor. Exp Cell Res 264: 29–41.
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T et al. (1998). Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95: 29–39.
Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW . (2000). High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res 60: 3605–3611.
Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH et al. (1997). Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15: 356–362.
Strong LC, Williams WR, Tainsky MA . (1992). The Li–Fraumeni syndrome: from clinical epidemiology to molecular genetics. Am J Epidemiol 135: 190–199.
Sulis ML, Parsons R . (2003). PTEN: from pathology to biology. Trends Cell Biol 13: 478–483.
Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J et al. (1999). PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA 96: 6199–6204.
Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I et al. (1998). High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol 8: 1169–1178.
Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM . (1998). Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280: 1614–1617.
Tang Y, Eng C . (2006). PTEN autoregulates its expression by stabilization of p53 in a phosphatase-independent manner. Cancer Res 66: 736–742.
Tlsty TD, White A, Livanos E, Sage M, Roelofs H, Briot A et al. (1994). Genomic integrity and the genetics of cancer. Cold Spring Harb Symp Quant Biol 59: 265–275.
Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP . (2006). Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441: 523–527.
Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H et al. (2007). Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128: 141–156.
Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ et al. (2007). Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell 11: 555–569.
Vogelstein B, Lane D, Levine AJ . (2000). Surfing the p53 network. Nature 408: 307–310.
Waite KA, Eng C . (2002). Protean PTEN: form and function. Am J Hum Genet 70: 829–844.
Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D et al. (1997). Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res 57: 4183–4186.
Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z et al. (2007). NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128: 129–139.
Weinberg RA . (1992). The retinoblastoma gene and gene product. Cancer Surv 12: 43–57.
Weng L, Brown J, Eng C . (2001a). PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Hum Mol Genet 10: 237–242.
Weng LP, Brown JL, Baker KM, Ostrowski MC, Eng C . (2002). PTEN blocks insulin-mediated ETS-2 phosphorylation through MAP kinase, independently of the phosphoinositide 3-kinase pathway. Hum Mol Genet 11: 1687–1696.
Weng LP, Smith WM, Brown JL, Eng C . (2001b). PTEN inhibits insulin-stimulated MEK/MAPK activation and cell growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos complex formation in a breast cancer model. Hum Mol Genet 10: 605–616.
Whiteman DC, Zhou XP, Cummings MC, Pavey S, Hayward NK, Eng C . (2002). Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int J Cancer 99: 63–67.
Woiwode A, Johnson SA, Zhong S, Zhang C, Roeder RG, Teichmann M et al. (2008). PTEN represses RNA polymerase III-dependent transcription by targeting the TFIIIB complex. Mol Cell Biol 28: 4204–4214.
Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D . (1993). p21 is a universal inhibitor of cyclin kinases. Nature 366: 701–704.
Yin Y, Tainsky MA, Bischoff FZ, Strong LC, Wahl GM . (1992). Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell 70: 937–948.
Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M . (1991). Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352: 345–347.
Acknowledgements
We are grateful to Drs C Walsh and HB Lieberman for their critical comments on the manuscript. This work was supported by a grant from the National Institutes of Health (R01 CA102447 to YY).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Yin, Y., Shen, W. PTEN: a new guardian of the genome. Oncogene 27, 5443–5453 (2008). https://doi.org/10.1038/onc.2008.241
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.241
Keywords
This article is cited by
-
A rat study on the PTEN expression in ovarian tissue in PCOS and folliculogenesis
Scientific Reports (2023)
-
Hyperphosphorylated PTEN exerts oncogenic properties
Nature Communications (2023)
-
Thyroid findings in pediatric and adult patients with PTEN hamartoma tumor syndrome: A retrospective analysis, and literature review
Endocrine (2023)
-
PTEN-related risk classification models for predicting prognosis and immunotherapy response of hepatocellular carcinoma
Discover Oncology (2023)
-
USP13 genetics and expression in a family with thyroid cancer
Endocrine (2022)