Abstract
Hepatic fat and abdominal adiposity individually reflect insulin resistance, but their combined effect on glucose homeostasis in mid-pregnancy is unknown. A cohort of 476 pregnant women prospectively underwent sonographic assessment of hepatic fat and visceral (VAT) and total (TAT) adipose tissue at 11–14 weeks’ gestation. Logistic regression was used to assess the relation between the presence of maternal hepatic fat and/or the upper quartile (Q) of either VAT or TAT and the odds of developing the composite outcome of impaired fasting glucose (IFG), impaired glucose tolerance (IGT) or gestational diabetes mellitus at 24–28 weeks’ gestation, based on a 75 g OGTT. Upon adjusting for maternal age, ethnicity, family history of DM and body mass index (BMI), the co-presence of hepatic fat and quartile 4 (Q4) of VAT (adjusted odds ratio (aOR) 6.5, 95% CI: 2.3–18.5) or hepatic fat and Q4 of TAT (aOR 7.8 95% CI 2.8–21.7) were each associated with the composite outcome, relative to women with neither sonographic feature. First-trimester sonographic evidence of maternal hepatic fat and abdominal adiposity may independently predict the development of impaired glucose homeostasis and GDM in mid-pregnancy.
Similar content being viewed by others
Introduction
Obesity in pregnancy has many consequences, including maternal insulin resistance (IR) and gestational diabetes mellitus (GDM).1, 2 Non-alcoholic fatty liver disease (NAFLD) and elevated abdominal adiposity are manifestations of the metabolic syndrome.3 Insulin resistance leads to a failure to suppress free fatty acids, which in turn, along with proinflammatory cytockines liberated from visceral adipose tissue (VAT), results in NAFLD.4, 5 We have previously shown that VAT and total adipose tissue (TAT) are each associated with IR in early pregnancy6 and impaired glucose homeostasis in mid-pregnancy,7 independent of BMI and other conventional risk factors for GDM.
While VAT and TAT are a reflection of IR, the co-presence of elevated abdominal AT and hepatic fat has not been evaluated in relation to dysglycemia and GDM at 24–28 weeks’ gestation, the recommended time to screen for GDM. Accordingly, we tested the hypothesis that non-diabetic women with sonographic evidence of hepatic fat and elevated VAT or TAT in early pregnancy would be at highest risk of dysglycemia and GDM in mid-pregnancy.
Research design and methods
This prospective cohort study was completed at a general obstetrics outpatient clinic at St Michael’s Hospital in Toronto, Ontario, Canada. The study was approved by the Research Ethics Board of St Michael’s Hospital, and participants provided written informed consent.
Healthy women aged 18 years and older were eligible for the study entry if they had a viable singleton pregnancy at 11–14 weeks’ gestation. To reduce confounding effects, we excluded women with known pre-pregnancy DM (prior GDM), a prior history of polycystic ovarian syndrome, metformin use, ovulation induction or other infertility treatment, use of corticosteroids for autoimmune conditions or other medical co-morbidities on medications, or any chronic or pregnancy-specific disorder that might affect liver function, such as viral hepatitis.
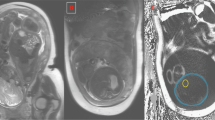
Visceral abdomen tissue and TAT depth quartiles (Q) were each determined by ultrasound at 11–14 weeks' gestation (at the time of routine assessment of fetal nuchal translucency), using a reliable and validated protocol, as described elsewhere.5, 6 Sonographic assessment of hepatic fat (present or absent) was determined in a standardized fashion in the sagittal plane, which allows single image capture of the liver and adjacent right kidney. Two sonographers independently reviewed the hard copies of each ultrasound image and used a semi-quantitative scoring method to assess hepatic fat based on the presence of any of the following: (i) diffusely increased echogenic (‘bright’) liver relatively greater than the right kidney (that is, ‘hepato-renal contrast’), and/or (ii) impaired visualization (blurring) of the portal and hepatic veins.5 This approach has a within-observer reliability of 0.95 and a between-observer reliability of 0.95, a sensitivity for NAFLD of 60–95%, and a specificity of 84–100%.3 Measurements were obtained using a Phillips IU22 and a GE E8 ultrasound machineswith either a 5–2 MHz or 9 MHz probe.6
At the time of the ultrasound measures, we collected information about maternal age, ethnicity (Caucasian, Black, South Asian, East Asian or Other), self-reported pre-gestational height and weight, and a history of type 2 DM among a first-degree relative. Weight at 11–14 weeks was directly measured using a calibrated scale.
A 75 g glucose tolerance test was performed at 24–28 weeks gestation, after an overnight fast. We defined impaired glucose homeostasis as a composite of impaired fasting glucose (IFG: fasting glucose⩾5.3 mmol l−1), impaired glucose tolerance (IGT: 1 h glucose⩾10.6 mmol l−1 or 2 h glucose⩾8.9 mmol l−1) or GDM (⩾ 2 abnormal glucose values, namely, fasting⩾5.3 mmol l−1, 1 h⩾10.6 mmol l−1 and/or 2 h⩾8.9 mmol l−1).2
Data analysis
Multivariable logistic regression analysis was used to assess the association between the combination of maternal hepatic fat and/or Q4 of VAT and the composite outcome. Participants without sonographic evidence of hepatic fat and within Q1–Q3 of VAT served as the referent. The same model was created for the combination of hepatic fat and Q4 of TAT. Odds ratios were adjusted (aOR) for maternal age (continuous in years), ethnicity (Caucasian vs Non-Caucasian), any first-degree relative with type 2 DM, measured BMI at 11–14 weeks gestation (continuous in kg m−2) and change in BMI from 11–14 weeks to 24–28 weeks’ gestation (continuous in kg m−2).
Statistical analyses were performed using SAS (version 9.1.3; SAS Institute, Cary, NC, USA).
Results
There were 476 women analyzed, at a mean (s.d.) age of 32.9 (4.8) years (Table 1). BMI at 11–14 weeks gestation ranged from 17.2 to 49.9 kg m−2, with a mean (s.d.) of 25.1 (5.1) kg m−2. A total of 50 out of 476 women (10.5%) developed the composite of IFG, IGT or GDM.
The combined presence of hepatic fat and Q4 of VAT was associated with a higher risk of impaired glucose homeostasis (aOR 6.5, 95% CI: 2.3 to 18.5) (Table 2). In contrast, those women without hepatic fat on ultrasound but whose VAT was at Q4, had a marginally higher risk of the composite outcome (aOR 2.3, 95% CI: 1.0 to 5.4). For the combined presence of hepatic fat and Q4 of TAT on ultrasound, the aOR was 7.8 (95% CI 2.8 to 21.7).
Conclusions
First-trimester maternal hepatic fat, in combination with a high VAT or TAT depth, predicted impaired glucose homeostasis at 24–28 weeks’ gestation, independent of maternal age, ethnicity, family history of type 2 DM or maternal BMI.
Our study strength was the inclusion of a relatively large, multi-ethnic sample of women, followed prospectively from the first trimester of pregnancy. In addition, use of a standardized sonographic protocol, coinciding with prenatal measurement of fetal nuchal translucency, afforded a practical timepoint to assess AT and hepatic fat. As a limitation, ultrasonography was only able to identify hepatic fat in a qualitative manner, and could not detect small amounts of hepatic steatosis and the stages of NAFLD.3, 8
VAT appeared to be an independent predictor of type 2 DM and the metabolic syndrome.9, 10 Excess VAT involves greater release of free fatty acids into the portal circulation along with proinflammatory cytokines,9, 10 and manifests as hepatic fat and inflammation that defines the spectrum of NAFLD.9 NAFLD has been shown to be more prevalent in non-pregnant women with previous GDM (38%, 95% CI: 28 to 47) than those without previous GDM (17%, 95% CI: 10 to 24).11 Using linked administrative datasets in Sweden, women with a coded diagnosis of NAFLD before pregnancy had a higher risk of GDM than those without a coded diagnosis of NAFLD (adjusted relative risk 2.78, 95% CI: 1.25–6.15).12 Thus, preliminary evidence suggests that women with excess hepatic fat may be at higher risk of GDM.
Research on objectively measured central obesity and impaired glucose homeostasis in pregnancy is also limited. We previously found that VAT and TAT respectively contributed to 23 and 25% of the variance in IR during the first trimester of pregnancy.6 More recently, we showed that Q4 of TAT depth, and especially Q4 of VAT depth, were associated with dysglycemia and GDM.7 What was not known is whether there is an additive effect when high VAT or TAT depth are considered in conjunction with the presence of hepatic fat. The current study findings suggest that there is an additive effect, and the reason may be that individuals with high VAT or TAT in conjunction with hepatic fat may have a pathological predisposition to IR. Among the 355 women in the VAT Q1–3 group, 48 (13.5%) had identified hepatic fat, whereas, among the 28 women in the VAT Q4 group, only 11 (39.3%) had sonographic signs of hepatic fat, resulting in a six times higher odds.
Assessing for hepatic fat in combination with VAT or TAT, using first-trimester ultrasonography, may offer a promising method of early detection of IR and a woman’s predisposition to GDM. The sonographic measurement of hepatic fat and adipose tissue in early pregnancy could be a cost-effective method easily added to routine, early antenatal screening. Women identified as having elevated hepatic fat and VAT or TAT during routine antenatal care at 11–14 weeks’ gestation, could then be identified in early pregnancy as having a predisposition to IR, and accordingly, might be offered early glucose testing, in addition to efficacious interventions that reduce their risk of GDM and related perinatal complications — something that must be proven within large scale prospective studies.13
References
Solomon CG, Willett WC, Carey VJ, Rich Edwards J, Hunter DJ, Colditz GA et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997; 278: 1078–1083.
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Can J Diabetes 2008; (Suppl): 172–180.
Charatcharoenwitthaya P, Lindor KD . Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis 2007; 11: 37–54.
Tiikkainen M, Tamminen M, Hakkinen AM, Bergholm R, Vehkavaara S, Halavaara J et al. Liver-fat accumulation and insulin resistance in obese women with previous gestational diabetes. Obes Res 2002; 10: 859–867.
Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007; 102: 2708–2715.
De Souza LR, Kogan E, Berger H, Alves JG, Lebovic G, Retnakaran R et al. Abdominal Adiposity and Insulin Resistance in Early Preganncy. J Obstet Gynaecol Can 2014; 36: 969–975.
De Souza LR, Berger H, Retnakaran R, Maguire JL, Nathens AB, Connelly PW et al. First-trimester maternal abdominal adiposity predicts dysglycemia and gestational diabetes mellitus in midpregnancy. Diabetes Care 2016. in press 39: 61–64.
Sadeeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123: 745–750.
Kantartzis K, Machann J, Schick F, Fritsche A, Haring HU, Stefan N . The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia 2010; 53: 882–889.
Despres JP . Abdominal obesity as important component of insulin-resistance syndrome. Nutrition 1993; 9: 452–459.
Forbes S, Taylor-Robinson SD, Patel N, Allan P, Walker BR, Johnston DG . Increased prevalence of non-alcoholic fatty liver disease in European women with a history of gestational diabetes. Diabetologia 2011; 54: 641–647.
Hagstrom H, Hoijer J, Ludvigsson JF, Bottai M, Ekbom A, Hultcrantz R et al. Adverse outcomes of pregnancy in women with non-alcoholic fatty liver diease. Liver Int 2016; 36: 268–274.
Simmons D, van Poppel MN . DALI consortium. UPBEAT, RADIEL, and DALI: what's the difference? Lancet Diabetes Endocrinol 2015; 3: 761.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR Funding Reference Number 126080). Dr Ray is supported by an Applied Research Chair in Reproductive and Child Health Services and Policy Research from CIHR. We gratefully thank our ultrasonographers who carried out the measurements in this study, Sharon MacGarvie, Carrie Crerar, Louisa Loizides and Meredith Hood.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
De Souza, L., Berger, H., Retnakaran, R. et al. Hepatic fat and abdominal adiposity in early pregnancy together predict impaired glucose homeostasis in mid-pregnancy. Nutr & Diabetes 6, e229 (2016). https://doi.org/10.1038/nutd.2016.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nutd.2016.39
This article is cited by
-
Adiponectin deficiency induces hepatic steatosis during pregnancy and gestational diabetes in mice
Diabetologia (2022)
-
The possible role of visceral fat in early pregnancy as a predictor of gestational diabetes mellitus by regulating adipose-derived exosomes miRNA-148 family: protocol for a nested case-control study in a cohort study
BMC Pregnancy and Childbirth (2021)
-
Obesity during pregnancy results in maternal intestinal inflammation, placental hypoxia, and alters fetal glucose metabolism at mid-gestation
Scientific Reports (2019)
-
Advances in assessing body composition during pregnancy
European Journal of Clinical Nutrition (2018)