Abstract
Background:
Mormordica charantia (bitter melon) has been investigated for lowering plasma glucose in patients with diabetes mellitus (DM). Previous data has offered inconclusive and inconsistent results about the benefits of bitter melon in patients with DM. Our current project aims to determine whether bitter melon has a favorable effect in lowering plasma glucose in patients with DM.
Methods:
We searched PubMed, EMBASE and the Cochrane Library from inception to July 2013 without any language restrictions for randomized controlled trials (RCTs) evaluating bitter melon to no treatment in patients with type 1 or type 2 diabetes. Study selection, data extraction and validity of each article were independently assessed by two investigators. Articles were appraised for proper random sequence generation, allocation concealment, blinding, selective reporting and completeness of outcomes reporting to assess the risk for biases. The glycemic results of each RCT were analyzed to yield weighted mean differences (WMDs) and 95% confidence intervals (CIs).
Results:
A total of four RCTs, each with 40–66 participants, followed between 4 and 12 weeks were identified in this meta-analysis. Overall risk of bias for each article included was determined to be unclear. In total, 208 participants with type 2 DM (mean age of 56.5 years) were evaluated. Compared with no treatment, bitter melon did not significantly lower A1C (WMD −0.13%, 95% CI −0.41 to 0.16) nor fasting plasma glucose (FPG) 47 (WMD 2.23 mg dl−1, 95% CI −14.91 to 19.37).
Conclusions:
Bitter melon supplementation compared with no treatment did not show significant glycemic improvements on either A1c or FPG.
Similar content being viewed by others
Introduction
In 2010 it was estimated that the world prevalence of diabetes among adults (aged 20–79 years) was 6.4% (285 million adults).1 Perhaps even more concerning is that diabetes is projected to affect approximately 7.7% of the world’s population (439 million adults) by 2030.1 Type 1 diabetes mellitus (T1DM) is considered as an autoimmune disease resulting in absolute insulin deficiency. Consequently, patients who are diagnosed with T1DM require therapy with exogenous insulin. In contrast, type 2 diabetes mellitus is characterized by a decline in β-cell function and worsening insulin resistance.2 Therapy for type 2 diabetes mellitus focuses around improving glucose tolerance through diet, exercise and oral anti-diabetic medications. Of the individuals affected with diabetes, approximately 95% are diagnosed with type 2 diabetes mellitus.3
Despite the advances in modern medicine, DM continues to be the most common endocrine metabolic disorder and the disease is rapidly increasing worldwide affecting all parts of the world.4 Many of the available therapies (that is, biguanides, sulfonylureas, glinides and insulin) have serious adverse effects associated with their use making the search for a more effective and safer hypoglycemic agent an important area of investigation.4 It is currently estimated that up to one-third of patients with DM use some form of supplemental medicine.
Momordica charantia (bitter melon) is a popular fruit used as a supplementary agent to treat DM in the populations of Asia, South America, India and East Africa. The exact mechanism of M. charantia is unknown; however, the active components charatin, vicine and polypeptide p is thought to be structurally similar to human insulin.5 Thus, some of the proposed mechanism of M. charantia includes insulin-like effects, increased insulin secretion, tissue glucose uptake, liver muscle glycogen synthesis and decrease glucose absorption.
There is insufficient evidence in the literature to make a definitive conclusion about the effects of bitter melon on glucose control. Current trials evaluating bitter melon on glycemic control has led to conflicting results and hence a systematic review of the available evidence was conducted. The objective of this paper is to conduct a systematic review and meta-analysis to evaluate the use of bitter melon on glycemic outcomes in patients with DM.
Materials and methods
Study selection
We performed a systematic literature search of PubMed, EMBASE and Cochrane Central Register of Controlled Trials from database start through September 2014 without language restriction to identify randomized controlled trials which compared bitter melon to no treatment in patients with type 1 or type 2 DM. Search terms included combining MeSH and text keywords for bitter melon (M. charantia), type 1 and type 2 diabetes, glucose levels and hemoglobin A1c. Studies were included if they were randomized controlled trials that evaluated bitter melon versus no treatment in either type 1 or type 2 diabetes and report results on A1c or fasting plasma glucose (FPG). Study selection was conducted by two independent investigators with disagreements resolved by discussion or a third investigator.
Data extraction and quality assessment
The following data were extracted from the included studies: baseline characteristics, study duration, inclusion and exclusion criteria, bitter melon dose and specific product used, results on A1c and FPG. Data were extracted by two independent investigators with disagreements resolved by discussion or a third party. Validity assessment was performed by two investigators independently using the Cochrane Risk of Bias Tool.6 This checklist includes six validity questions covering the following domains: random sequence generation, allocation concealment, blinding, blinding of outcome assessment, incomplete data reporting and selective reporting. Each item was scored as a low, unclear or high risk of bias (Figure 2). Upon analyzing each domain in the Cochrane Risk of Bias tool, an overall risk of bias score of either low, unclear, or high per trial was derived. A low risk of bias score was assigned to a study if most of the information from that study was at low risk of bias. An unclear risk of bias score was assigned to a study if most of the information from that study was at low or an unclear risk of bias, and a high risk of bias score was assigned to a study if the proportion of information from that study at high risk of bias was sufficient to affect the interpretation of the results.6
Data synthesis and analysis
The mean changes in FPG and A1c from baseline were treated as continuous variables, and the weighted mean differences and accompanying 95% confidence intervals were pooled using a DerSimonian and Laird random-effects model.7 Changes from baseline in outcomes were extracted from trials; in instances where changes were not reported directly, they were calculated from end-of-study and baseline results. Outcome data were extracted as analyzed in that specific trial without any additional adjustment for potential losses to follow-up. As suggested by Follmann et al.,8 we assumed a correlation coefficient of 0.5 between initial and final values. The statistical analysis was performed by using StatsDirect statistical software, version 2.7.8 (Altrincham, Chesire, UK). A P-value <0.05 was considered statistically significant for all analyses. Statistical heterogeneity was planned to be assessed using the I2 statistic, but too few studies were available to assess.9 Publication bias was planned to be assessed using visual inspection of funnel plots and Egger’s weighted regression statistics, but too few studies were available to assess.10
Results
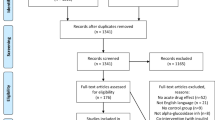
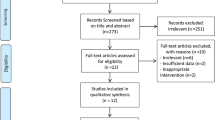
The search strategy yielded 50 nonduplicate citations for screening. (Figure 1) Six full-text articles were screened and three trials (n=187) met all inclusion criteria. Only two trials reported usable data for meta-analysis of A1c11, 12 (Table 1) and two trials reported meta-analyzable data for FPG11, 13 (Table 1). Patients were randomized to be treated with either bitter melon or control (dosing range 1–6 g per day) in various dosage forms (tablets or capsules) for a period of 4–12 weeks (Table 1). Although our search strategy allowed for the inclusion of patients with type 1 diabetes, all studies that met inclusion criteria evaluated patients with type 2 diabetes. The risk of bias assessment for individual validity components is presented in Figure 2.
Quantitative data synthesis
Upon meta-analysis, there was no statistically significant association between bitter melon usage and A1c (weighted mean difference −0.13%, 95% confidence interval −0.41 to 0.16) or FPG (weighted mean difference 2.23 mg dl−1, 95% confidence interval −14.91 to 19.37 mg dl−1) when compared with control (Figure 3a and b, respectively). Very few studies were available for each analysis to allow for the assessment of heterogeneity or publication bias.
Forest plot depicting result from meta-analysis of trials evaluating the effects of bitter melon on A1c and FPG versus placebo based on the year of publication. Note: The squares represent the pooled results of that study in addition to all studies preceding it. Errors bars represent 95% Cls and the diamond represents the overall pooled results. The solid vertical line extending upward from 0 is the null value.
Discussion
Previous data has offered mixed conclusions about the benefits of bitter melon in patients with diabetes. Although it has been suggested that bitter melon promotes post-meal insulin secretion, thereby improving glycemic response, this has only been showed in a single-dose experiment without a control group.14 This mechanism of activity may require the presence of viable pancreatic β cells to secrete insulin in order for hypoglycemic activity to take place. It is possible that the patients evaluated in randomized controlled trials have limited β-cell function, decreasing their ability to respond to bitter melon. In this meta-analysis, pooling together the available evidence found no statistically significant effect for the use of bitter melon versus control on A1c or FPG.
The small number of available trials on this topic is a limitation of this analysis. The small sample of trials may be insufficient to detect statistically significant effects; because of this, it is unclear whether bitter melon truly exerts no effect or if the analysis is underpowered. The variety of doses and dosage forms of bitter melon contribute to the potential heterogeneity of the evidence. The duration of study is also limited, with the longest trial at 12 weeks and the others only 4 weeks long; however, the outcome A1c generally requires at least 8–12 weeks of therapy to detect changes.15, 16, 17, 18 It is likely that this current evidence could not adequately detect effects on A1c if this effect were to exist. The effects of bitter melon on A1c may require a longer duration of therapy before any potential benefits on A1c are seen. Owing to the limited report of adverse effects, we could not meta-analyze safety parameters related to bitter melon. However, one trial reported that the most common complaints were about gastrointestinal discomfort.11
Overall, the evidence regarding the use of bitter melon on glycemic outcomes in patients with DM is inconclusive. Additional evidence in a larger sample of patients evaluated over a longer duration of time is needed to determine whether bitter melon is truly ineffective in patients with DM.
References
Shaw JE, Sicree RA, Zimmet PZ . Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14.
Fonseca VA . Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009; 32: S151–S156.
National Diabetes Information Clearinghouse (NDIC). National Diabetes Statistics 2011, http://diabetes.niddk.nih.gov/dm/pubs/statistics/#fast Accessed July 2013.
Patel DK, Prasad SK, Kumar R, Hemalatha S . An overview on anti-diabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed 2012; 2: 320–330.
Basch E, Garbardi S, Ulbricht C . Bitter melon (Momordica charantia: A review of efficacy and safety. Am J Health-Syst Pharm 2003; 60: 356–359.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et alCochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928.
DerSimonian R, Laird N . Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188.
Follmann D, Elliott P, Suh I, Cutler J . Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992; 45: 769–773.
Higgins JP, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560.
Egger M, Davey SG, Schneider M, Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
Dans AML, Villarruz MVC, Jimeno CA, Javelosa MAU, Chua J, Bautista R et al. The effect of Momordica charantia capsule preparation on glycemic control in type 2 diabetes mellitus needs further studies. J Clin Epidemiol 2007; 60: 554–559.
Zanker KS, Mang B, Wolters M, Hahn A . Personalized diabetes and cancer medicine: a rationale for anti-diabetic nutrition (bitter melon) in a supportive setting. Curr Cancer Ther Rev 2012; 8: 66–77.
John AJ, Cherian R, Subhash HS, Cherian AM . Evaluation of the efficacy of bitter gourd (Momordica charantia as an oral hypoglycemic agents–a randomized controlled clinical trial. Indian J Physiol Pharmacol 2003; 47: 363–365.
Lim ST, Jimeno CA, Razon-Gonzales EB, Velasquiz EN . The MOCHA DM study: the effect of Momordica charantia tablets on glucose and insulin levels during the post prandial state among patients with type 2 diabetes mellitus. Phillipine J Int Med 2010; 48: 19–25.
Sacks DB . Measurement of hemoglobin A1c. Diabetes Care 2012; 35: 2674–2680.
Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS . Systematic Review of Herbs and Dietary Supplements for glycemic control in diabetes. Diabetes Care 2003; 26: 1277–1294.
Fuangchan A, Sonthisombat P, Seubnekarn T, Chanouan R, Chotchaisuwat P, Sirigulsatein V et al. Hypoeglycemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patients. J Ethnopharmacol 2011; 134: 422–428.
Leung L, Birtwhistle R, Kotecha J, Hannah S, Cuthbertson S . Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): a mini review. Br J Nutr 2009; 102: 1703–1708.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work has been presented as a poster at the American Society of Health-Systems Pharmacists Midyear Clinical Meeting in Orlando, Fl in December 2013.
Disclaimer
All authors certify that none of the material in this manuscript has been previously submitted or published and is not currently under review elsewhere. All authors attest to authorship and agree to release copyright upon acceptance and publication.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Yin, R., Lee, N., Hirpara, H. et al. The effect of bitter melon (Mormordica charantia) in patients with diabetes mellitus: a systematic review and meta-analysis. Nutr & Diabetes 4, e145 (2014). https://doi.org/10.1038/nutd.2014.42
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nutd.2014.42
This article is cited by
-
Anti-diabetic properties of traditional herbal concoction containing Eleutherine palmifolia (L.) Merr., Momordica charantia L., and Syzygium polyanthum (Wight.): a bibliometric analysis
Food Production, Processing and Nutrition (2023)
-
Systematic review and meta-analysis protocol for efficacy and safety of Momordica charantia L. on animal models of type 2 diabetes mellitus
Systematic Reviews (2020)
-
Safety and efficacy of Momordica charantia Linnaeus in pre-diabetes and type 2 diabetes mellitus patients: a systematic review and meta-analysis protocol
Systematic Reviews (2018)
-
Acute effects of a beverage containing bitter melon extract (CARELA) on postprandial glycemia among prediabetic adults
Nutrition & Diabetes (2017)