Abstract
Background:
Short sleep and weight gain are inversely related. Sleep deprivation acutely increases food intake but little is known about eating behavior in chronically sleep-deprived, obese individuals.
Objective:
To characterize the relationship between sleep, food intake and alcohol consumption under free-living conditions in obese, chronically sleep-deprived individuals.
Design:
Cross-sectional study of a cohort of obese men and premenopausal women.
Subjects:
A total of 118 obese subjects (age: 40.3±6.7 years; 91 females/27 males; body mass index 38.7±6.4 kg m−2).
Measurements:
Energy, macronutrient, alcohol and caffeine intake assessed by 3-day food records. Sleep duration estimated by actigraphy. Respiratory disturbance index assessed by a portable device.
Results:
Subjects slept 360.7±50.2 min per night and had a total energy intake of 2279.1±689 kcal per day. Sleep duration and energy intake were inversely related (r=−0.230, P=0.015). By extrapolation, each 30-min deficit per day in sleep duration would translate to an ∼83 kcal per day increase in energy intake. In addition, sleep apnea was associated with a shift from carbohydrate to fat intake. Alcohol intake in subjects consuming >3.5 g of alcohol per day (N=41) was inversely related to sleep duration (r=−0.472, P=0.002).
Conclusions:
Shorter sleep duration and obstructive sleep apnea are associated with higher energy, fat and alcohol intakes in obese individuals. The importance of this study relies on the population studied, obese subjects with chronic sleep deprivation. These novel findings apply to the large segment of the US population who are obese and sleep-deprived.
Similar content being viewed by others
Introduction
Based on the thermodynamic law, obesity results from a state of positive energy balance in which energy intake exceeds energy expenditure over a prolonged period of time. In the past decades, there has been a dramatic increase in the prevalence of obesity.1 The reasons for such epidemic are unknown and are the subject of an ongoing debate.2, 3 In the search for hidden causative factors, it was noted that the obesity epidemic has been paralleled by a concomitant shortening in self-reported sleep and by an increase in the prevalence of obstructive sleep apnea (OSA).2 Thus, we and others have hypothesized that these pervasive contemporaneous epidemics, sleep deprivation and obesity, may be linked together in causal, reverberating circuits.2
Most of the information available for the effects of sleep deprivation on food intake is derived from acute studies conducted in healthy lean male volunteers. Experimentally induced acute sleep deprivation increases appetite in healthy lean humans.4 In rodents, sleep deprivation changes the hypothalamic expression of several neuropeptides, including the orexogenic neuropeptide Y and the anorexogenic proopiomelanocortin.5 Less is known about food intake in chronically sleep-deprived human subjects, especially in those with obesity. The information about long-term association between sleep duration and body weight stems from large, epidemiological studies, mostly designed for other purposes.6, 7, 8 Short sleep increases the risk of obesity, with a 0.35 kg m−2 change in body mass index (BMI) per hour of sleep change, as indicated by a meta-analysis.9 Another recent meta-analysis quantified the impact of the drive to eat on alcohol intake, sleep deprivation and television watching;10 all three factors had an impact, with large size effects for alcohol intake (1.02) and sleep deprivation (0.49), and a smaller effect (0.2) for television watching. Data from the Quebec Family Study, a cohort study for the etiology of obesity, indicated that short sleep duration, together with low dietary calcium intake and high disinhibited eating behavior is associated with a higher BMI.11 Excessive fat intake and irregular meal patterns relate to both short sleep duration and OSA.12, 13, 14, 15, 16, 17 Of note, acute experiments reveal little about long-term adaptations, and epidemiological studies lack mechanistic information and cannot establish causality.18
Ethanol is a sleep-promoting gamma-amino-butyric acid receptor agonist.19 Ethanol relaxes the upper airway dilator muscles, increases upper airway resistance and may promote OSA in susceptible subjects.20 Heavy drinking is associated with short sleep duration, as indicated by the 2004–2006 National Health Institute Interview.21 Whether moderate alcohol consumption is related to sleep alterations is not well established. This is an important question, if one keeps in mind that moderate alcohol consumers comprise a large proportion of the American population.22
Our aim was to characterize the relationships between sleep characteristics, nutritional habits and alcohol intake in a sample of chronically sleep-deprived obese men and women. The importance of our study resides in the fact that for the first time the relationship between sleep and dietary intake was studied in such a population.
Subjects and methods
Study cohort
The analyses reported here are based on the baseline evaluation of a cohort of obese men and premenopausal women enrolled in a sleep extension study.23 In this randomized, controlled trial, subjects were randomized in a 2:1 ratio to an Intervention Group and to a Comparison Group and were followed for 1 year (efficacy phase). Subjects in the Intervention Group were encouraged to progressively extend sleep duration and to improve sleep quality in a non-pharmacological fashion by lifestyle modifications. An individualized plan was developed at the randomization visit by the study participant with the help of the study team and progresses were followed with short outpatient visits at monthly intervals. The Comparison Group was seen at the same time points and received information about the standard of care. At the end of 1 year, each subject, including those originally randomized to the Comparison Group, was invited to extend sleep duration and subjects were continued to be followed with less frequent visits for up to additional 3 years (effectiveness phase). Inclusion criteria included BMI of 29–55 kg m−2 and ages of 18–50 years. We advertised for subjects sleeping less than 6.5 h per night by self-assessment. Subjects were excluded if they were postmenopausal, regularly used sleep medications, consumed more than 500 mg of caffeine per day and/or had poorly controlled depression or anxiety, diabetes, hypertension or other systemic disease. Additional details of this study have been reported elsewhere.23 The study was conducted at the NIH Clinical Center in Bethesda under NIDDK protocol 06-DK-0036, is listed in ClinicalTrials.gov (identifier: NCT00261898), and was approved by the Scientific Review Board and the Institutional Review Board of the NIDDK.
Assessments
Energy and alcohol intake
Subjects were given verbal and written instructions to record their food and beverage intake for 3 consecutive days (2 weekdays and 1 weekend day, preferably Thursday, Friday and Saturday). Food records were reviewed by registered dietitians and health technicians using three-dimensional food models and other visual aids. Dietary intake data were analyzed using the Nutrition Data System for Research software versions 2007 through 2010, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN.24 Daily macronutrient, caffeine, alcohol and energy intakes were determined. Eating occasions were defined as eating episodes, which contained at least 20 kcal, and were consumed at least 30 min apart or in a different location.
Sleep measures
Habitual sleep was estimated subjectively by sleep diary and objectively by wrist actigraphy (Actiwatch-64, Mini Mitter/Respironics/Philips, Bend, OR, USA). A device similar to a wristwatch was worn continuously for 14 days. The Actiwatch-64 has been validated against polysomnography with excellent correlations in various patient populations.25 We based our analyses on two measures: night-time sleep and sleep efficiency (percentage of time in bed spent sleeping).
Sleep apnea was assessed using a portable screening device (Apnea Risk Evaluation System, Advanced Brain Monitoring, Inc., Carlsbad, CA, USA). This device estimates the respiratory disturbance index (RDI), which is the number of apneas and hypopneas per hour of sleep. This device has been validated with high sensitivity and specificity against polysomnography.26 Participants completed the Pittsburgh Sleep Quality Index, a validated 21-items questionnaire with inquiries about sleep, including perceived quality over the past month.27 Pittsburgh Sleep Quality Index scores range from 0 to 21, with higher scores indicating worse sleep quality. Scores were dichotomized at ⩽5 or >5, which conventionally indicates poor sleep quality. Subjective sleep duration was derived from Question Four: how many hours of sleep the participant usually gets per night. Daytime sleepiness was assessed by the Epworth Sleepiness Scale (ESS), a validated eight-item questionnaire.28 ESS scores range from 0 to 24, higher scores representing increased daytime sleepiness, while scores >10 indicate an excessive sleepiness.
Anthropometrics
Height was measured to the nearest centimeter using a wall mounted stadiometer (SECA 242, SECA North America East, Hanover, MD, USA) and weight was measured using a stand-on-scale in a hospital gown to the nearest 1/10th of a kg (SR555 SR Scales, SR Instruments, Inc, Tonawanda, NY, USA). Circumference measurements were done using a non-stretch measuring tape in triplicate to the nearest 1 mm. Waist circumference was measured at the uppermost lateral border of iliac crest at the end of a normal expiration. If this site could not be determined, the maximum circumference was measured at or near the level of the umbilicus. Neck circumference was measured at the minimal circumference with the head in the Frankfort Horizontal Plane.
Body composition
Body composition was measured by dual energy X-ray absorptiometry (Lunar iDXA, General Electric, Chicago, IL, USA).
Statistical analysis
Descriptive statistics were calculated first for the cohort as a whole and then separately by gender. Tests used to compare men and women included Student’s t-test for difference in mean values, Mann–Whitney U-test for skewed variables, Fisher exact test and Pearson Chi-square test for difference in counts and frequency. The Kolmogorov–Smirnov test was used to assess normality; logarithmic transformations were employed for skewed variables (e.g. RDI values, alcohol and caffeine intakes). Pearson (r) and Spearman (rho) correlation coefficients were employed for Gaussian and skewed variables, respectively. The degree of agreement between subjective and objective sleep duration estimates was quantified by computing a concordance correlation coefficient.
Simple regression analyses of sleep and diet components selected a priori were conducted based on pre-defined hypotheses. Specific predictors from these regression analyses showing a P-value less than 0.10 in univariate analysis were then included into multiple linear regression models. Age, gender, race and BMI were used as covariates.
Data are presented as a mean value±s.d. Analyses were performed using SAS (version 9.1.3, SAS Institute Inc., Cary, NC, USA), JMP (version 8.0, SAS Institute) and SPSS (version 19 IBM SPSS North America, Chicago, IL, USA).
Results
Demographic, anthropometric and sleep characteristics
A total of 118 individuals out of the 126 subjects randomized between 22 January 2007 and 28 June 2011 for whom dietary information was available were the object of this analysis. Age was 40.3±6.7 years and BMI was 38.7±6.4 kg m−2 (Table 1). Three quarters of the participants were women and two-thirds were self-identified as non-Hispanic blacks. Men had a larger waist and neck circumference, a larger fat-free mass and a lower percentage of body fat than women. On average, our sample had short sleep duration and decreased sleep efficiency, as indicated by both total sleep time, (∼6 h) and sleep efficiency (∼80%). Sleep apnea was highly prevalent: only 42% of the participants had a normal (<5) RDI score. We noted the following gender differences: sleep apnea (RDI >5) was more prevalent, 80% vs 50%, and more severe in men than women. By actigraphy, women slept on average 13 min longer than men. The coefficient of concordance between sleep duration by actigraphy and sleep diary was significant, 0.413 (95% confidence interval: 0.244–0.557, P<0.001, N=90) (Figure 1). Nevertheless, sleep duration by sleep diary and sleep duration by actigraphy were different (sleep diary: +23 min 95% confidence interval: 12–33 min, P<0.001) (Figure 1).
The average total energy intake was ∼2280 kcal/day; 48% of calories derived from carbohydrates, 14% from proteins and 36% from fat. On average, there were four eating occasions per day (Table 2). Alcohol intake had a non-Gaussian distribution with a mean of 43 kcal per day. Of the 118 subjects, 77 reported consuming less than 3.5 g of alcohol per day, roughly the equivalent of ¼ of an alcoholic beverage per day. The remaining 41 alcohol drinkers, had a consumption of less than two drinks per day, with only 7 subjects consuming more than four drinks per day (Figure 2). Total energy intake was higher in men compared with women, with no differences in macronutrient composition. Caffeine intake was ∼40% higher in men than women (P=0.013; Table 2).
Relationship between sleep and total energy intake
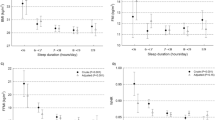
Sleep duration by actigraphy was inversely related to total energy intake, whereras severity of sleep apnea, as indicated by RDI scores, was directly related to total energy intake (Figures 3a and b). Thus, sleeping less and having more episodes of sleep apnea was associated with a higher total energy intake. Furthermore, RDI was inversely related to carbohydrate intake (r=−0.309; P=0.001; N=98) and directly related to fat intake (r=0.239; P=0.017; N=98), both expressed as percent of total energy intake. When assessed by gender, these relationships persisted only in women: the carbohydrate intake was inversely (r=−0.396; P<0.001; N=78), while the fat intake was directly (r=0.325; P=0.017; N=78) related to RDI. Neither caffeine intake nor the number of eating occasions were related to any of the sleep variables (P>0.05).
Relationships between total energy intake and sleep duration by actigraphy (a) and RDI score (b) and relationship between sleep duration by actigraphy and alcohol intake in the subsample of subjects consuming more than ¼ alcoholic beverage per day (c). Note use of ‘Safe Log’ (that is, log10(1+RDI)) for the logarithmic transformation of RDI scores in the analyses and graph (b).
To further explore the relationship between sleep and dietary intake, we constructed several multivariate models (Table 3). Sleep duration by actigraphy and RDI accounted for ∼9% of the variability in total energy intake (Model 1). After adjustment for age and gender, the variability rose to 21% (Model 2). Results remained similar after adding BMI to the model (Model 3). When race/ethnicity was included (Model 4), the variability in total energy intake accounted for by the model became 26%. Based on Model 4, a 30-min decrease in sleep would correspond to 83-kcal increase (30 × 2.76=82.8) in energy intake. Age was inversely related to total energy intake, so that a 10-year increase in age would correspond to ∼236 kcal decrease in energy intake. In each one of the four models, sleep apnea, as characterized by RDI scores, was inversely related to carbohydrate intake and directly related to fat intake.
Relationships between sleep and alcohol intake
Alcohol intake and sleep parameters were correlated in the 41 subjects consuming more than ¼ alcoholic beverage per day (3.5 g per day) (Table 4). Alcohol intake was inversely related to total sleep time (Figure 3c). In the multivariate analyses, alcohol intake explained ∼22% of the variability in sleep duration (Model 1) (Table 4). Incremental adjustments for age and gender (Model 2), plus BMI (Model 3), plus race/ethnicity (Model 4), increased the variability accounted for by the models to 33%.
Discussion
To our knowledge, this is the first report on the association between sleep and dietary/alcohol intake in a real life setting in obese, chronically sleep-deprived subjects. We assessed sleep duration subjectively and objectively, determined the presence and severity of sleep apnea, and estimated dietary intake using food records for 3 consecutive days. Energy intake in women was ∼15% higher than that of an average American woman of comparable age, whereas in men the reported intake was similar to the national average.29 Diet composition in both genders was similar to the pattern reported for a typical American adult.29
Together, short sleep duration and sleep apnea accounted for a substantial amount of the variability in energy intake, a parameter notoriously influenced by a variety of different factors.30 In our sample, a 30-min decrease in sleep duration would correspond to an increase of 83 kcal per day. Sleeping less than 6.5 h per night, instead of the recommended 7.5 h per night would translate into an excess consumption of 1200 kcal per week, and, if no compensation occurs, a weight gain of ∼8 kg in 1 year. Because of the cross-sectional nature of our report, we neither can claim causality, nor can we account for its direction. In addition, given the known presence of long-term adaptive mechanisms, our extrapolation should be taken with caution and verified in prospective studies.
In healthy lean humans, acute sleep restriction induces food intake31, 32, 33 and stimulates hunger via increasing plasma ghrelin (a ‘hunger’ hormone) levels and decreasing plasma leptin (a ‘satiety’ hormone).4 We have previously reported that leptin concentrations were not related to sleep measurements in our study.8 Our findings were in agreement with another short-term study of sleep deprivation,34 observing no changes in leptin levels, but they were at variance with another short-term study conducted in healthy lean male subjects4
Sleep curtailment and the modern environment, which is characterized by endless opportunities to eat, potentiate each other in a vicious circle.35 Excessive food intake, especially consumed late in the day, could be responsible for digestive difficulties and consequent sleep-time reduction.36, 37, 38 Furthermore, several studies have identified single nutrients (that is, glucose, amino acids), capable of resetting or phase-shifting circadian rhythms of the internal clock.39 Finally, inadequate sleep, excessive energy intake and obesity are associated with lower socioeconomic status.40, 41 A cross-sectional survey of 2670 women, ages 18–55 years, reported that several socioeconomic factors including age, education, depressive symptoms, marital and employment status were associated with sleep characteristics such as short sleep and inadequate sleep42
Severity of sleep apnea was associated with a shift in macronutrient consumption. Total energy intake and fat intake are strong determinants of energy balance and predictors of weight gain. High-fat diets are more likely than high carbohydrates and protein diets to lead to weight gain.43, 44 We observed a direct relationship between RDI and carbohydrate and fat intake only in women, possibly because of the smaller number of males in our sample. These findings of greater carbohydrate intake in women with sleep apnea are similar to a cross-sectional study of teenage Saudi girls reporting greater ∼16% greater carbohydrate intake in subjects sleeping less than 5 h vs those sleeping more than 7 h.45
In agreement with our findings, the APPLE Study showed that fat consumption was higher in women with severe sleep apnea.46 Analyzing food intake in subjects before and after treatment with OSA would be helpful in understanding the cause–effect link between OSA and eating habits and assist in implementing therapy.47
Alcohol intake and total sleep time were inversely related in our subjects consuming at least ¼ serving of alcohol per day, the equivalent of 1–2 alcoholic beverages per week. Daily alcohol intake accounted for ∼1/5 of the variability in sleep duration. Each increase of one drink (∼14 g of alcohol) would result in a 30-min decrease in sleep duration. Thus, alcohol intake, even in small quantities may interfere with sleep. Up to two or three standard drinks before bedtime favor sleep initiation, an effect that vanishes after a few days.48 In a large clinical series of 2933 healthy Japanese individuals, aged 46–60 years, drinkers were less likely to maintain sleep than nondrinkers.49 Chronic alcohol abuse disrupts sleep even in abstinent alcoholics.50, 51, 52, 53 Insufficient and poor quality sleep induce chronic stress54 and subjects with chronic anxiety and post-traumatic stress disorder often use alcohol to relieve stress.55 Similarly, chronically sleep-deprived subjects may attempt to self-medicate themselves with alcohol. Additional factors of the relationship of alcohol and sleep include alcohol doses, the times of consumption relative to sleep, and the time of the day. Alcohol consumption increases upper airway resistance,20 especially during the first 2 h after ingestion.
It is well known that the accuracy of dietary intake data is limited and depends on many factors, including the rigor of the dietary data collection procedures as well as personal characteristics of the subjects, including gender and BMI. Obese women tend to under-report energy intake compared with predicted energy intake estimated from predictive equations.56 The extent to which obese subjects under-report their dietary intake vs actually restricting their intake when asked to record their food intake, remains to be determined. We recently reported55 that the subjects in this study had a measured average resting energy expenditure of 1700 kcal per day. An empirical rule calls for multiplying the measured resting energy expenditure by a factor of 1.4 in sedentary individuals to estimate the total daily energy expenditure. Thus, the mean energy intake reported in this study, ∼2300 kcal per day was very close to the theoretical was very close to the theoretical energy expenditure reported previously.56
Our study merits include a prospectively assembled, and well-characterized sample. Consistent with the epidemiology of sleep deprivation, our sample included a large proportion of minorities and women. Sleep was characterized by an array of objective measures and standardized questionnaires. Our analyses were, however, cross-sectional and the study design did not include a group of obese, non-sleep-deprived subjects. Dietary intake was assessed via 3-day food record collection, a method known to be associated with under-reporting in obese, female populations. However, the current food records were in agreement with previously reported data of predicted energy needs in this group of subjects as assessed using measured resting energy expenditure, indicating that the rigorous methods for collection of this data may have improved the quality of this data. Unfortunately, validation of this data with doubly labeled water, the gold standard for assessing dietary intake was impractical for this study. More than two-thirds of the subjects in our sample did not consume alcohol in a regular fashion, and the amount of alcohol intake was assessed together with food intake, rather than by a specific questionnairie. Therefore, the relatively small number of subjects consuming alcohol and the method of estimation of alcohol intake limited the extension of our findings to the general population. Finally, our sample size of 118 subjects may have had a limited statistical power to detect some of the more subtle associations.
Approximately 36%, roughly 80 millions, of adult Americans, are obese1 and 29% get insufficient rest or sleep.57 Based on the 2005–2008 NHANES data, the prevalence of self-reported sleep apnea is ∼9%.58 It is estimated that up to 80% of individuals with moderate or severe sleep apnea, a treatable condition by continuous positive airway pressure (CPAP) or similar devices, currently go undiagnosed.59 A conservative estimate calls for at least 18 million of adult Americans with sleep apnea, most of them undiagnosed and untreated. Long-term compliance with CPAP is known to be poor, due to its limited tolerability. In summary, this study provides quantitative estimates of the associations between sleep and food and alcohol intake in obese subjects in free-living conditions. Even small amounts of alcohol intake can disrupt sleep duration and quality. Sleep duration and OSA are related to total energy intake and severity of OSA is associated with a shift from carbohydrate to fat intake.
Our findings have important bearings on long-term weight regulation for the increasing number of the US population, who are obese, chronically sleep deprived, and suffer from sleep apnea. Clinical approaches to this population should be individualized and informed by determination of dietary intake and assessment of sleep apnea. Sleep apnea, a major determinant of food intake in these subjects, should be diagnosed and treated. Novel devices with a better tolerability profile should be developed.
References
Flegal KM, Carroll MD, Kit BK, Ogden CL . Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012; 307: 491–497.
Cizza G, Rother KI . Beyond fast food and slow motion: weighty contributors to the obesity epidemic. J Endocrinol Invest 2012; 35: 236–242.
McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 2009; 49: 868–913.
Spiegel K, Tasali E, Penev P, Van Cauter E . Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141: 846–850.
Koban M, Le WW, Hoffman GE . Changes in hypothalamic corticotropin-releasing hormone, neuropeptide Y, and proopiomelanocortin gene expression during chronic rapid eye movement sleep deprivation of rats. Endocrinology 2006; 147: 421–431.
Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension 2007; 50: 693–700.
Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep 2004; 27: 661–666.
Knutson KL, van Cauter E . Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci 2008; 1129: 287–304.
Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008; 31: 619–626.
Chapman CD, Benedict C, Brooks SJ, Birgir Schiöth H . Lifestyle determinants of the drive to eat: a meta-analysis. Am J Clin Nutr 2012; 96: 492–497.
Chaput JP, Leblanc C, Pérusse L, Després JP, Bouchard C, Tremblay A . Risk factors for adult overweight and obesity in the Quebec Family Study: have we been barking up the wrong tree? Obesity 2009; 10: 1964–1970.
Grandner MA, Kripke DF, Naidoo N, Langer RD . Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med 2010; 11: 180–184.
Hicks RA, McTighe S, Juarez M . Sleep duration and eating behaviors of college students. Percept Mot Skills 1986; 62: 25–26.
Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S . An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci 2002; 21: 115–120.
Shi Z, McEvoy M, Luu J, Attia J . Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond) 2008; 32: 1835–1840.
Spruyt K, Sans Capdevila O, Serpero LD, Kheirandish-Gozal L, Gozal D . Dietary and physical activity patterns in children with obstructive sleep apnea. J Pediatr 2010; 156: 724–730.
Weiss A, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S . The association of sleep duration with adolescents' fat and carbohydrate consumption. Sleep 2010; 33: 1201–1209.
Cizza G, Skarulis M, Mignot E . A link between short sleep and obesity: building the evidence for causation. Sleep 2005; 28: 1217–1220.
Lu J, Greco MA . Sleep circuitry and the hypnotic mechanism of GABAA drugs. J Clin Sleep Med 2006; 2: S19–S26.
Lam JC, Sharma SK, Lam B . Obstructive sleep apnoea: definitions, epidemiology & natural history. Indian J Med Res 2010; 131: 165–170.
Schoenborn CA, Adams PF Sleep duration as a correlate of smoking, alcohol use, leisure-time physical inactivity, and obesity among adults: United States, 2004-2006 CDC Available at: http://www.cdc.gov/nchs/data/hestat/sleep04-06/sleep04-06.htm Accessed June 10 2012.
Breslow RA, Guenther PM, Juan W, Graubard BI . Alcoholic beverage consumption, nutrient intakes, and diet quality in the US adult population, 1999-2006. J Am Diet Assoc 2010; 110: 551–562.
Cizza G, Marincola P, Mattingly M, Williams L, Mitler M, Skarulis M et al. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials 2010; 7: 274–285.
Schakel SF . Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products - A research perspective. J Food Comp Anal 2001; 14: 315–322.
Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H . The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills 1997; 85: 207–216.
Westbrook PR, Levendowski DJ, Cvetinovic M, Zavora T, Velimirovic V, Henninger D et al. Description and validation of the apnea risk evaluation system: a novel method to diagnose sleep apnea-hypopnea in the home. Chest 2005; 128: 2166–2175.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213.
Nguyen AT, Baltzan MA, Small D, Wolkove N, Guillon S, Palayew M . Clinical reproducibility of the Epworth Sleepiness Scale. J Clin Sleep Med 2006; 2: 170–174.
U.S. Department of Agriculture. Agricultural Research Service. 2010. Energy intakes: percentages of energy from protein, carbohydrate, fat, and alcohol, by gender and age, What We Eat in America, NHANES 2007-2008 Available at: http://www.ars.usda.gov/ba/bhnrc/fsrg Accessed 10 June 2012.
Sharma AM, Padwal R . Obesity is a sign—over-eating is a symptom: an aetiological framework for the assessment and management of obesity. Obes Rev 2010; 11: 362–370.
Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D . Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010; 91: 1550–1559.
Nedeltcheva AV, Kessler L, Imperial J, Penev PD . Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009; 94: 3242–3250.
St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 2011; 94: 410–416.
Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B . A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res 2008; 17: 331–334.
Cizza G, Requena M, Galli G, de Jonge L . Chronic sleep deprivation and seasonality: implications for the obesity epidemic. J Endocrinol Invest 2011; 34: 793–800.
Fox M, Barr C, Nolan S, Lomer M, Anggiansah A, Wong T . The effects of dietary fat and calorie density on esophageal acid exposure and reflux symptoms. Clin Gastroenterol Hepatol 2007; 5: 439–444.
Kellow JE, Delvaux M, Azpiroz F, Camilleri M, Quigley EM, Thompson DG . Applied principles of neurogastroenterology: physiology/motility sensation. Gastroenterology 2006; 130: 1412–1420.
Jung HK, Choung RS, Talley NJ . Gastroesophageal reflux disease and sleep disorders: evidence for a causal link and therapeutic implications. J Neurogastroenterol Motil 2010; 16: 22–29.
Froy O . Metabolism and circadian rhythms—implications for obesity. Endocr Rev 2009; 31: 1–24.
Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep 2009; 32: 731–736.
Popkin BM, Adair LS, Ng SW . Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012; 70: 3–21.
Kachikis AB, Breitkopf CR . Predictors of sleep characteristics among women in southeast Texas. Women Health Issues 2012; 22: e99–109.
Bray GA, Popkin BM . Dietary fat intake does affect obesity!. Am J Clin Nutr 1998; 68: 1157–1173.
Savage JS, Marini M, Birch LL . Dietary energy density predicts women's weight change over 6 y. Am J Clin Nutr 2008; 88: 677–684.
Al-Disi D, Al-Daghri N, Khanam L, Al-Othman A, Al-Saif M, Sabico S et al. Subjective sleep duration and quality influence diet composition and circulating adipocytokines and ghrelin levels in teen-age girls. Endocr J 2010; 10: 915–923.
Vasquez MM, Goodwin JL, Drescher AA, Smith TW, Quan SF . Associations of dietary intake and physical activity with sleep disordered breathing in the Apnea Positive Pressure Long-Term Efficacy Study (APPLES). J Clin Sleep Med 2008; 4: 411–418.
McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess 2009; 13, iii-iv, xi-xiv, 1-119 143–274.
Stein MD, Friedmann PD . Disturbed sleep and its relationship to alcohol use. Subst Abus 2005; 26: 1–13.
Kadono M, Hasegawa G, Shigeta M, Nakazawa A, Ueda M, Fukui M et al. Joint effect of alcohol and usual sleep duration on the risk of dysglycemia. Sleep 2007; 10: 1341–1347.
Brower KJ . Insomnia, alcoholism and relapse. Sleep Med Rev 2003; 7: 523–539.
Smith C, Smith D . Ingestion of ethanol just prior to sleep onset impairs memory for procedural but not declarative tasks. Sleep 2003; 26: 185–191.
Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WD . The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med 2007; 8: 215–221.
Boscarino JA, Kirchner HL, Hoffman SN, Sartorius J, Adams RE . PTSD and alcohol use after the World Trade Center attacks: a longitudinal study. J Trauma Stress 2011; 24: 515–525.
Mitler MM, Dawson A, Henriksen SJ, Sobers M, Bloom FE . Bedtime ethanol increases resistance of upper airways and produces sleep apneas in asymptomatic snorers. Alcohol Clin Exp Res 1988; 12: 801–805.
de Jonge L, Zhao X, Mattingly MS, Zuber SM, Piaggi P, Csako G et al. Poor sleep quality and sleep apnea are associated with higher resting energy expenditure in obese individuals with short sleep duration. J Clin Endocrinol Metab 2012; 97: 2881–2889.
Poppitt SD, Swann D, Black AE, Prentice AM . Assessment of selective under-reporting of food intake by both obese and non-obese women in a metabolic facility. Int J Obes Relat Metab Disord 1998; 22: 303–311.
Centers for Disease Control and Prevention (CDC). Perceived insufficient rest or sleep among adults - United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58: 1175–1179.
Plantinga L, Rao MH, Schillinger D . Prevalence of self-reported sleep problems among people with diabetes in the United States 2005–2008. Prev Chronic Dis 2012; 9: E76.
Lee W, Nagubadi S, Kryger MH, Mokhlesi B . Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med 2008; 2: 349–364.
Acknowledgements
This study was fully supported by the National Institutes of Health (NIH), Intramural Research Program: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This study was supported by the Intramural Program of the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) and the Clinical Center, National Institutes of Health (NIH). This study was conducted under the NIDDK protocol 06-DK-0036 and is listed in ClinicalTrials.gov (identifier: NCT00261898). We would like to thank in alphabetical order the following colleagues for their scientific advice and critical suggestions in the development and conduct of the study protocol: Karim Calis, Janet Gershengorn, Gregor Hasler, Emmanuel Mignot, Susan Redline, Kristina I Rother, Terry Phillips, Nancy Sebring, Duncan Wallace, Robert Wesley, Elizabeth Wright. We would also like to thank the members of the study team: Peter Bailey, Laide Bello, Meredith Coyle, Paula Marincola, Patrick Michaels, Svetlana Primma, Angela Ramer, Rebecca Romero, Megan Sabo, Tanner Slayden, Sara Torvik, Sam Zuber, Elizabeth Widen and Lyda Williams. The bioinformatics support of Frank Pierce (Esprit Health) is gratefully acknowledged. Finally we are grateful to all of our enthusiastic study participants.
Author Contributions
GCi and GCs designed research, MSM, ABC, GCi, conducted research, GG, PP and LDJ analyzed data or performed statistical analysis, GG, PP, LDJ, AP, FS, GCi and GCs wrote paper (only authors who made a major contribution); GG, GCi and GCs had primary responsibility for final content.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Galli, G., Piaggi, P., Mattingly, M. et al. Inverse relationship of food and alcohol intake to sleep measures in obesity. Nutr & Diabetes 3, e58 (2013). https://doi.org/10.1038/nutd.2012.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nutd.2012.33
Keywords
This article is cited by
-
The relationship between sleep duration, sleep quality and dietary intake in adults
Sleep and Biological Rhythms (2020)
-
Alcohol as Food/Calories
Current Addiction Reports (2016)
-
Stress, Sleep, Depression and Dietary Intakes Among Low-Income Overweight and Obese Pregnant Women
Maternal and Child Health Journal (2015)