Abstract
Background:
Obesity is associated with a prothrombotic state, which may contribute to the increased risk of thrombotic events.
Objective:
To assess the effects of (pre)adipocyte-derived adipokines on fibrinogen, plasminogen activator inhibitor-1 (PAI-1) and tissue factor (TF) production by hepatocytes.
Methods:
HepG2 hepatocytes were incubated with conditioned media (CM) derived from preadipocytes and adipocytes, which had been untreated or prestimulated with tumor necrosis factor (TNF)-α, interleukin (IL)-1β or IL-6. After 24 h, supernatants and cell lysates were harvested for measurement of fibrinogen, PAI-1 and TF.
Results:
(Pre)adipocyte CM significantly enhanced the production of PAI-1 by HepG2 cells 2.5- to 4.4-fold. CM from cytokine-stimulated (pre)adipocytes significantly induced fibrinogen secretion 1.5- to 4.2-fold. TF production was not affected by the CM. After specific depletion of TNF-α, IL-1β or IL-6 from the CM, IL-6 was shown to be the most prominent stimulus of fibrinogen secretion and IL-1β of PAI-1 secretion. In addition, fibrinogen, PAI-1 and tissue factor production was evaluated by direct stimulation of HepG2 cells with TNF-α, IL-1β or IL-6. IL-6 enhanced fibrinogen synthesis 4.3-fold (P<0.01), whereas IL-1β induced PAI-1 production 5.0-fold (P<0.01). Gene expression analyses showed that TNF-α and IL-1β stimulate the adipocyte expression of TNF-α, IL-1β and IL-6. Cytokine stimulation of adipocytes may thus have induced an inflammatory response, which may have stimulated fibrinogen and PAI-1 production by HepG2 cells more potently.
Conclusions:
SGBS (pre)adipocytes release cytokines that increase the production of fibrinogen and PAI-1 by HepG2 cells. IL-6 and IL-1β produced by (pre)adipocytes were the strongest inducers of fibrinogen and PAI-1 secretion, respectively.
Similar content being viewed by others
Introduction
Abdominal obesity is strongly related to insulin resistance, type 2 diabetes mellitus, non-alcoholic fatty liver disease and vascular complications.1, 2, 3 In addition to metabolic derangements, resulting in insulin resistance and low-grade inflammation, obesity is characterized by abnormalities in hemostasis, coagulation and fibrinolysis, contributing to arterial and venous thrombosis.4 The prothrombotic state in obesity is reflected by platelet dysfunction, elevated levels of fibrinogen, tissue factor (TF), factor VII and the fibrinolysis inhibitor plasminogen activator inhibitor-1 (PAI-1).5, 6, 7, 8
It has been suggested that dysfunctional adipose tissue with a proinflammatory microenvironment has an important role in the development of the prothrombotic state.7, 9, 10, 11 The adipose tissue secretoma, which consists of a wide range of functionally different proteins together called adipokines, may exert autocrine, paracrine and endocrine effects.12 These adipokines may originate not only from the stromal vascular fraction, infiltrating inflammatory cells but also from the adipocyte fraction. Adipose tissue is directly involved in coagulation and fibrinolysis by adipocyte secretion of TF, PAI-1 and possibly thrombin-activatable fibrinolysis inhibitor.8, 13, 14, 15, 16 Adipose tissue may also influence platelet function by secretion of adipokines eventually leading to platelet activation.17, 18
Owing to the close anatomical relation between visceral adipose tissue (VAT) and the liver, the primary source of coagulation factors, it has been suggested that adipokines and free fatty acids secreted by VAT into the portal vein may influence the hepatic production of coagulation proteins. The close interaction between VAT and the liver through the portal vein was demonstrated by Fontana and co-workers,11 who demonstrated 50% higher portal concentrations compared with the systemic circulation. The amount of IL-6 in the portal vein was associated with the amount of C-reactive protein derived from the liver.11 Although the association between the VAT–liver interaction and inflammation has been established, the pathophysiological mechanisms by which adipose tissue may induce a hypercoaguable and hypofibrinolytic state are largely unknown.
In this study, we investigated the effects of conditioned media (CM) from adipocytes and preadipocytes on the production of fibrinogen, PAI-1 and TF by HepG2 hepatoma cells. We hypothesized that proinflammatory cytokines/adipokines present in the CM from adipocytes and preadipocytes stimulate the production of fibrinogen, PAI-1 and TF by hepatocytes. Confirmation of this hypothesis would support the concept of an adipose tissue–liver axis in which adipose tissue influences the hepatic production of coagulation factors and fibrinolysis inhibitors by releasing adipokines into the portal circulation.
Methods
SGBS cell culture
SGBS cells were generously provided by Dr Martin Wabitsch from the University of Ulm (Ulm, Germany) and differentiated to adipocytes using a procedure modified from previous publications.19, 20 Briefly, SGBS cells were grown to confluence in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 Ham’s (Gibco Life Technologies, Blijswijk, The Netherlands) supplemented with 10% fetal bovine serum (Gibco Life Technologies), 33 μM biotin, 17 μM pantothenate, 100 μg ml−1 streptomycin and 62.5 μg ml−1 penicillin (Gibco Life Technologies) at 37 °C and 5% CO2. At 2 days post-confluence, SGBS cells were stimulated to adipogenic differentiation with serum-free growth medium supplemented with 20 nM insulin, 200 pM triiodothyronine, 1 μM cortisol, 500 μM isobutylmethylxanthine, 25 nM dexamethasone, 0.01 mg ml−1 human transferrin and 2 μM rosiglitazone. After 4 days, the medium was replaced by differentiation medium without isobutylmethylxanthine, dexamethasone and rosiglitazone. The degree of differentiation was assessed microscopically 10 days after differentiation counting the ratio between preadipocytes and differentiated adipocytes. Also, the levels of peroxisome proliferator-activated receptor-γ gene expression were measured as a marker of differentiation showing a 14-fold increase in peroxisome proliferator-activated receptor-γ gene expression in differentiated SGBS adipocytes compared with preadipocytes. When at least 80% of the SGBS cells displayed an adipocytic phenotype, the medium was replaced by serum-free medium. Local inflammation in adipose tissue was mimicked by replacing the serum-free medium by serum-free medium containing different concentrations of the proinflammatory cytokines (tumor necrosis factor (TNF)-α or interleukin (IL)-1β) during 24 h. Unstimulated controls were SGBS cells incubated in the medium without cytokines during 24 h. Preadipocyte conditioned medium was harvested in a similar manner, except that the medium was harvested from undifferentiated SGBS cells, which had reached near confluence.
HepG2 cell culture
HepG2 human hepatoma cells were obtained from the European Collection of Cell Cultures (no. 85011430). The cell line was grown in 80 cm2 tissue-culture flasks (Nunc, Roskilde, Denmark) using Eagle’s minimal essential medium (Life Technologies Ltd, Paisley, UK) supplemented with 10% fetal bovine serum (Life Technologies) and 2 mmol l−1 L-glutamine (Life Technologies), 5 ml of non-essential amino acids (Life Technologies) and 4 mg l−1 fungizone (Bristol-Meyers Squibb, Woerden, The Netherlands). Before the experiments, HepG2 cells were propagated and maintained for at least three passages. In the experiments, cells were seeded in 48-well plates (Corning Costar; Corning Inc., Corning, NY, USA) and grown to confluence at 37 °C under 5% CO2. To determine the number of cells per well, cells were detached using trypsin solution and afterwards cells were counted in suspensions using a Bürker–Türk chamber.
Incubation
Before incubation with CM, HepG2 cells were cultured with serum-free medium during 24 h. Serum-free medium was then replaced by the differential CM from preadipocytes and adipocytes and incubated for 24 h. After incubation of HepG2 cells with CM, supernatants were harvested and fibrinogen and PAI-1 were measured. TF was measured in HepG2 cell lysates, after lysis of HepG2 cells with Triton X-100 (2%) solution.
Immunoassays
Enzyme-linked immunosorbent assays (ELISAs) were conducted for fibrinogen (Kordia–Biopool, FG-EIA Affinity Biologicals Inc., Leiden, The Netherlands), PAI-1 (PAI-1 antigen ELISA reagent kit, no. TC11070; Technoclone GmbH, Vienna, Austria), TF (Imubind Tissue Factor ELISA kit, product no. 845; American Diagnostica GmbH, Pfungstadt, Germany), IL-1β (Pelikine compact human IL-1β assay M1934; Sanquin Reagents, Amsterdam, The Netherlands), IL-6 (Pelikine IL-6 assay M1916; Sanquin Reagents) and TNF-α (Pelikine compact human TNF-α kit M1923; Sanquin Reagents) according to the manufacturer’s instructions. For fibrinogen a standard curve was constructed from normal hemostasis reference plasma (no. 50720; Kordia-Biopool). Standard curves for IL-1β, IL-6, TNF-α, PAI-1 and TF were constructed from reference samples supplied with the kits. All parameters were measured in cell culture supernatants, except for TF that was measured in lysates of cultured HepG2 cells.
Cytokine depletion
For selective cytokine depletion of (pre)adipocyte CM, the corresponding antibodies were precoated to the wells of a 96-well plate during at least 24 h. After washing the wells, 1000 μl of CM was incubated in these wells during 24 h. After incubation, cytokine-depleted CM were collected and stored at −20 °C until further analysis. Depletion efficiency was determined by testing differential predepletion and postdepletion CM in corresponding cytokine ELISAs. Using the described method of cytokine depletion from the CM, 85% of IL-1β, 82% of IL-6 and 80% of TNF-α could be removed from the CM, as was determined with ELISA measurements before and after cytokine depletion (data not shown).
Quantitative reverse transcription-polymerase chain reaction
SGBS preadipocytes were differentiated as described above. SGBS were lysed using Qiazol Lysis Reagent (no. 79306; Qiagen, Venlo, The Netherlands). After chloroform treatment, total RNA was isolated from the aqueous phase using the RNeasy Lipid Tissue Mini Kit (no. 74804; Qiagen). Yield and purity of isolated RNA were determined using photospectrometric analysis (BioSpec-nano; Shimadzu Biotech, Seattle, WA, USA). cDNA was synthesized using Superscript II Reverse Transcriptase (Invitrogen, Blijswijk, The Netherlands), according to the manufacturer’s protocol. Expression levels of human IL-1β, IL-6 and TNF-α were determined by real-time polymerase chain reaction (PCR) using the LightCycler (Roche Applied Science, Almere, The Netherlands). Briefly, the relative expression of the housekeeping gene 36B4 was used to calculate the relative expression of IL-1β, IL-6 and TNF-α. The PCR reactions were performed using the DNA Master SYBR-green 1 kit (Roche Applied Science) and contained 5.0 μl of 1:10 diluted cDNA, 0.25 pmol μl−1 primer, DNA master SYBR-green I solution and MgCl2 (3.5 mM). For each sample, PCR reactions were carried out in duplicate. Primer sequences were as follows: human IL-1β—sense primer, 5′-TACCTGTCCTGCGTGTTGAA-3′, antisense primer, 5′-TCTTTGGGTAATTTTTGGGATCT-3′; human IL-6—sense primer, 5′-TACCCCCAGGAGAAGATTCC-3′, antisense primer, 5′-TTTCAGCCATCTTTGGAAGG-3′; human TNF-α—sense primer, 5′-TCTTCTCGAACCCCGAGTGA-3′, antisense primer, 5′-CCTCTGATGGCACCACCAG-3′; human 36B4—sense primer, 5′-CGGGAAGGCTGTGGTGCTG-3′, antisense primer, 5′-GTGAACACAAAGCCCACATTCC-3′.
Data analyses
Gene expression results are expressed as means±standard error of the mean (s.e.m.). Differences in quantitative measures were tested for significance using the unpaired two-tailed Student’s t-test. Significance was established when P<0.05.
Results
Effects of (pre)adipocyte CM on production of fibrinogen, PAI-1 and TF by HepG2 cells
To investigate changes in TF, fibrinogen and PAI-1 production induced by adipokines secreted by preadipocytes and adipocytes, HepG2 cells were cultured with CM derived from preadipocytes, differentiated adipocytes or control media for 24 h. To mimic adipose tissue inflammation, (pre)adipocytes were incubated with medium supplemented with TNF-α (1 or 10 ng ml−1) or in medium without the cytokine.
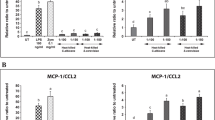
As presented in Figure 1, fibrinogen synthesis by HepG2 cells was not affected by adipocyte CM from unstimulated adipocytes. However, adipocyte CM from adipocytes cultured with 1 ng ml−1 TNF-α for 24 h caused a significant 1.5-fold increase in fibrinogen production to a level of 1010±114 ng ml−1 (P<0.05). CM from preadipocytes stimulated with TNF-α (1 and 10 ng ml−1) induced a significant increase in fibrinogen production by HepG2 cells (2745±41 and 2251±382 ng ml−1, respectively, vs 654±26 ng ml−1 in the untreated HepG2 cells, P<0.01). Fibrinogen synthesis by HepG2 cells did not significantly increase after incubation with CM from unstimulated preadipocytes (1802±500 ng ml−1, P=0.08).
PAI-1 production by HepG2 cells was stimulated upon incubation with unstimulated and TNF-α-stimulated preadipocyte or adipocyte CM: CM from adipocytes caused a significant 2.5- to 3.0-fold increase in PAI-1 production compared with untreated HepG2 cells. In addition, preadipocyte CM induced a 3.2- to 4.4-fold increase in PAI-1 production.
There was no increase in TF production by HepG2 cells after incubation with either preadipocyte or adipocyte CM.
As a control, fibrinogen, PAI-1 and TF concentrations were also measured in the (pre)adipocyte CM before incubation with HepG2 cells; there was no significant contribution of adipocytes and preadipocytes to the levels of FG, PAI-1 or TF in the harvested conditioned medium (data not shown).
In conclusion, PAI-1 production by HepG2 cells is promoted by CM from unstimulated as well as stimulated adipocytes and preadipocytes. Significant increases in fibrinogen synthesis could be demonstrated only in CM derived from (pre)adipocytes after stimulation with TNF-α. Synthesis of TF was not altered by culturing HepG2 cells with any (pre)adipocyte CM.
Expression of proinflammatory cytokines and cytokine receptors in adipocytes
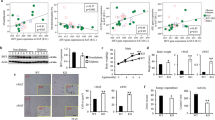
To evaluate the expression of the cytokines TNF-α, IL-1β, IL-6 in SGBS adipocytes after 24 h stimulation with TNF-α or IL-1β, quantitative RT–PCR analyses of gene expression were performed. Gene expression of IL-1β and IL-6 was significantly increased after stimulation with IL-1β (1 ng ml−1) (Figure 2a). Incubation of adipocytes with TNF-α (10 ng ml−1) for 24 h resulted in a significant increase in the gene expression of the proinflammatory cytokines TNF-α, IL-1β and IL-6 (Figure 2b).
The current data indicate that adipocyte stimulation with TNF-α and IL-1β induces expression of the proinflammatory cytokines TNF-α, IL-1β and IL-6, which may lead to increased production and secretion of these cytokines into the medium.
Effects of cytokine depletion on adipocyte conditioned medium-mediated production of fibrinogen and PAI-1 by HepG2 cells
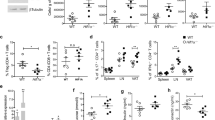
To evaluate the contribution of the adipocyte-produced proinflammatory cytokines TNF-α, IL-1β and IL-6 to the CM effects on prothrombotic factors (Figure 1), antibody-mediated depletion of these cytokines was performed. Depletion of IL-6 led to significant reductions in fibrinogen synthesis in CM derived from unstimulated adipocytes (regular CM vs IL-6-depleted CM: 347±2 vs 315±5 ng ml−1 (P<0.01)) and adipocytes stimulated with IL-1β (1 ng ml−1) (regular CM vs IL-6-depleted CM: 1829±211 vs 660±78 ng ml−1 (P<0.01)) (Figure 3A). Also, fibrinogen synthesis was reduced after depleting preadipocyte CM of IL-6: unstimulated preadipocyte regular CM vs IL-6-depleted CM, 1015±14 vs 506±24 ng ml−1 (P<0.001); TNF-α (1 ng ml−1) stimulated preadipocyte regular CM vs IL-6-depleted CM, 1709±230 vs 850±104 ng ml−1 (P<0.05); and TNF-α- (10 ng ml−1) stimulated preadipocyte regular CM vs IL-6-depleted CM, 1773±293 vs 958±54 ng ml−1 (P<0.05) (Figure 3a). Specific removal of IL-1β or TNF-α from the CM did not induce changes in fibrinogen synthesis (data not shown).
(A) Effects of (pre)adipocyte conditioned medium on the production of fibrinogen by HepG2 cells before and after removal of IL-6. The conditioned media (CM) were derived from either unstimulated adipocytes and preadipocytes (CM) or from adipocytes and preadipocytes stimulated with TNF-α (1 or 10 ng ml−1) and IL-1β (1 ng ml−1) during 24 h (n=3). (B) Effects of (pre)adipocyte conditioned medium on the production of PAI-1 by HepG2 cells before and after the removal of (a) IL-1β, (b) IL-6 or (c) TNF-α. The CM were derived from either unstimulated adipocytes and preadipocytes (CM) or from adipocytes and preadipocytes stimulated with TNF-α (1 or 10 ng ml−1) and IL-1β (1 ng ml−1) during 24 h (n=3).
PAI-1 concentrations generally decreased significantly after depletion of IL-1β from the CM: TNF-α-(1 ng ml−1) stimulated adipocyte regular CM vs IL-1β-depleted CM, 589±27 vs 429±17 ng ml−1 (P<0.01); TNF-α- (10 ng ml−1) stimulated adipocyte regular CM vs IL-1β-depleted CM, 700±19 vs 392±58 ng ml−1 (P<0.01); unstimulated preadipocyte regular CM vs IL-1β-depleted CM, 612±54 vs 447±27 ng ml−1 (P<0.05); and TNF-α- (10 ng ml−1) stimulated preadipocyte regular CM vs IL-1β-depleted CM, 1041±34 vs 504±88 ng ml−1 (P<0.01) (Figure 3B).
In general, there were no alterations in PAI-1 production after depletion of IL-6 from CM.
TNF-α depletion of (pre)adipocyte CM resulted in significant PAI-1 reductions in CM derived from unstimulated adipocytes: unstimulated adipocyte CM vs TNF-α-depleted CM, 463±22 vs 323±39 ng ml−1 (P<0.05).
In conclusion, depletion of predominantly IL-1β and, to a lesser extent, TNF-α in preadipocyte and adipocyte CM led to important reductions in PAI-1 production by HepG2 cells, whereas IL-6 depletion reduced fibrinogen production.
Direct effects of proinflammatory cytokines on fibrinogen, PAI-1 and TF production by HepG2 cells
Having established that depletion of IL-6 ablated CM-mediated induction of fibrinogen production, while IL-1β (and to lesser extent TNF-α) depletion reduced the effects on PAI-1 production (Figure 3), we wished to establish whether these proinflammatory cytokines are by themselves sufficient to alter prothrombotic factor production. Fibrinogen production was increased upon incubation with IL-6 (1, 10 and 100 ng ml−1) for 24 h, reaching an optimum at an IL-6 concentration of 10 ng ml−1 (2840±2 vs 654±26 ng ml−1 in untreated HepG2 cells). Incubation with IL-1β (1, 10 and 100 ng ml−1) for 24 h resulted in a small albeit significant decrease in fibrinogen synthesis by HepG2 cells (Figure 4). PAI-1 production was greatly enhanced by IL-1β treatment, with the strongest effect at 10 ng ml−1 (1574±78 vs 310±14 ng ml−1 in untreated HepG2 cells). IL-1β treatment resulted in a stronger increase of PAI-1 production than adding TNF-α and IL-6, which also increased PAI-1 levels. In agreement with the CM data (Figure 1), TF concentrations remained relatively constant after the addition of cytokines; only high-dose TNF-α (100 ng ml−1) and IL-1β (10 ng ml−1) induced significant increases in TF concentrations.
Thus, IL-6 and IL-1β, which are produced by adipocytes, can directly stimulate the production of fibrinogen and PAI-1, respectively, whereas TF production by HepG2 cells remains largely unaltered after direct addition of cytokines.
Discussion
As a model for investigating the potential of adipose tissue to influence the production of procoagulant factors by the liver, this study evaluated the effects of CM from preadipocytes and adipocytes on the production of fibrinogen, PAI-1 and TF by HepG2 cells in vitro. The current results demonstrate that IL-6 in (pre)adipocyte CM stimulates the production of fibrinogen by HepG2 cells and that IL-1β and TNF-α in (pre)adipocyte CM induce an increase in PAI-1 production by HepG2 cells (Figure 5). Although several cytokines such as TNF-α, IL-1β and IL-6 act as important inducers of TF production in different cell types,21, 22 only marginal increases in TF production by HepG2 cells after cytokine stimulation were observed in this study. There was no change in TF production after incubation with adipocyte or preadipocyte CM, suggesting that cytokine-induced production of TF is tissue/cell type dependent.
Potential mechanisms by which TNF-α- and IL-1β-stimulated adipocytes induce PAI-1 and fibrinogen production by liver cells: TNF-α stimulation leads to a strong increase in IL-1β and IL-6 gene expression in adipocytes, which may ultimately lead to elevated levels of IL-1β and IL-6 in the conditioned medium. IL-1β and IL-6 thereafter stimulate the production of, respectively, PAI-1 and fibrinogen in hepatocytes after incubation with the adipocyte CM. Stimulation with IL-1β will also lead to an enhanced adipocyte IL-1β and IL-6 gene expression. The increase of IL-1β gene expression by IL-1β is less potent than TNF-α, however. Stimulation of adipocytes with IL-1β may thus lead to enhanced production of PAI-1 and fibrinogen in hepatocytes after incubation with adipocyte CM.
This is the first study to evaluate and demonstrate the effects of adipocyte/adipose tissue CM on the synthesis of coagulation and fibrinolysis proteins by HepG2 hepatocytes. Other studies have reported metabolic alterations in hepatocytes induced by adipokine containing CM. Conditioned medium from in vitro differentiated adipocytes induced insulin resistance in HepG2 hepatocytes.23 Addition of resistin, which is primarily synthesized by macrophages in humans, induced stronger insulin resistance than CM alone, indicating that local adipose tissue inflammation with macrophage infiltration may have an important role in the pathogenesis of insulin resistance. Also, lipid accumulation in hepatocytes could be stimulated by culturing hepatocytes in conditioned medium derived from white adipose tissue explants. Monocyte chemoattractant protein-1 secreted by adipose tissue was shown to be responsible for the triglyceride accumulation in the hepatocytes, as incubation of conditioned medium with antimonocyte chemoattractant protein-1 antibodies prevented lipid accumulation.24 In addition to experiments using CM from adipose tissue or adipocytes, co-culture experiments using transwell systems demonstrated that insulin signaling in primary hepatocytes was significantly impaired by adipocyte-derived adipokines.25
Whereas there is a paucity of experimental data on the pathophysiological mechanisms linking adipose tissue with an increased hepatic synthesis of coagulation factors and fibrinolysis inhibitors, clinical studies have demonstrated a strong association between abdominal obesity and elevated plasma concentrations of TF, factor VII, fibrinogen and the fibrinolysis inhibitors PAI-1 and thrombin-activatable fibrinolysis inhibitor.8, 15, 26, 27, 28 Furthermore, independent of obesity and VAT amount, the extent of fat accumulation in the liver, known as non-alcoholic fatty liver disease, is strongly associated with a proinflammatory and prothrombotic state.29 Levels of the proinflammatory factors C-reactive protein and IL-6, as well as the prothrombotic factors fibrinogen and PAI-1, were shown to increase with more severe degrees of hepatic steatosis.29, 30 As it has been shown that VAT volume is directly associated with hepatic steatosis,31 these data underline the close metabolic interconnection between VAT and the liver.
Increasing adiposity accompanied by adipocyte hypertrophy leads to adipose tissue hypoxia and endoplasmic reticulum stress, which result in local inflammation, increased free fatty acid secretion and an altered adipokine secretion profile.10, 12, 32 Enhancement of the proinflammatory state may be induced by adipose tissue macrophages, which are recruited by increased local production of monocyte chemoattractant protein-1.33 Release of adipokines and proinflammatory factors by VAT in the portal circulation along with an increased free fatty acid flux may eventually lead to hepatic insulin resistance, intrahepatic triglyceride accumulation and alterations of the hepatic biosynthetic function with increased synthesis of acute phase proteins. Indeed, it has been shown previously in morbidly obese subjects that plasma concentrations of IL-6 were approximately 50% greater in the portal vein than in the radial artery.11 It also appeared that circulating IL-6 levels were specifically related to visceral adiposity.34 IL-6, which reaches the liver through the portal circulation, is a potent stimulus for the hepatic production of acute phase proteins such as C-reactive protein and fibrinogen,35 both of which are related to obesity.26, 36 Our data support the notion that inflamed adipose tissue may induce hepatic fibrinogen synthesis by secretion of IL-6.
However, the exact source of elevated PAI-1 plasma levels in obesity remains to be elucidated, as there are several sites of PAI-1 secretion37 in contrast to fibrinogen, which is predominantly synthesized in the liver. Although there have been repeated reports about PAI-1 production, especially by VAT,37 there was no significant contribution of the cultured adipocytes to PAI-1 levels in the adipocyte conditioned medium in this study. PAI-1 was thus primarily derived from HepG2 cells and IL-1β was the most prominent stimulus of PAI-1 production. Comparably, stimulatory effects of IL-1β on PAI-1 production have been observed in murine hepatic cells38 and subcutaneous human adipocytes.39 As IL-1β is produced by preadipocytes and adipocytes,40 and also by macrophages, adipose tissue-derived IL-1β may act as a regulatory cytokine for hepatic PAI-1 production.
Some limitations of the current experiments have to be considered. We acknowledge that the use of SGBS (pre)adipocytes and HepG2 hepatoma cell lines constitute a simplified model of the adipose tissue–liver interaction and that these cell lines are not fully representative of adipocytes and hepatocytes in vivo. Also, although SGBS adipocytes represent a widely used cell line to mimick adipocyte function in vitro, there are important morphological and functional differences between differentiated SGBS adipocytes and normal mature adipocytes in adipose tissue, which have to be acknowledged. The same limitation holds true for the use of HepG2 hepatoma cells as a model for hepatocytes. Therefore, the current data should be interpreted with caution and cannot be simply extrapolated to the complex in vivo interaction between adipose tissue and the liver. Furthermore, the use of selective antibodies to deplete cytokines from CM resulted in an effective but incomplete depletion of these cytokines. Although important effects of cytokine stimulation on fibrinogen and PAI-1 have been described in the current experiments, also other adipokines present in the CM may have had a significant contribution in stimulating the production of fibrinogen and PAI-1.
In addition, we acknowledge the fact that the concentrations of the exogenously added cytokines exceed physiological circulating concentrations in vivo, which may have elicited responses in the cultured SGBS cells that would not otherwise occur at lower concentrations. On the basis of previously published results on adipocyte stimulation with proinflammatory cytokines, the above-mentioned concentrations of TNF-α, IL-1β and IL-6 were used to create a local inflammatory response. Lower more physiological concentrations of proinflammatory cytokines most likely will not result in detectable metabolic changes using the current methods.
Finally, some of the exogenously added cytokines used to stimulate the (pre)adipocytes may have been carried over onto the HepG2 cells, inducing additional production of proinflammatory cytokines by these cells, which may have led to increased production of fibrinogen and PAI-1.
In conclusion, proinflammatory cytokines in CM from preadipocytes and adipocytes lead to increased production of fibrinogen and PAI-1 by human hepatocytes. IL-6 and IL-1β in the CM were the strongest inducers of, respectively, fibrinogen and PAI-1. These data support the concept that adipose tissue may enhance the hepatic production of coagulation proteins by secreting proinflammatory cytokines and adipokines in the portal circulation, thus causing a hypercoagulable state.
References
Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case–control study. Lancet 2005; 366: 1640–1649.
Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB . Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 2008; 117: 1658–1667.
Kotronen A, Yki-Jarvinen H . Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008; 28: 27–38.
Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008; 28: 1039–1049.
Godsland IF, Crook D, Proudler AJ, Stevenson JC . Hemostatic risk factors and insulin sensitivity, regional body fat distribution, and the metabolic syndrome. J Clin Endocrinol Metab 2005; 90: 190–197.
Meilahn EN, Cauley JA, Tracy RP, Macy EO, Gutai JP, Kuller LH . Association of sex hormones and adiposity with plasma levels of fibrinogen and PAI-1 in postmenopausal women. Am J Epidemiol 1996; 143: 159–166.
Faber DR, de Groot PG, Visseren FL . Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev 2009; 10: 554–563.
Eriksson P, Reynisdottir S, Lonnqvist F, Stemme V, Hamsten A, Arner P . Adipose tissue secretion of plasminogen activator inhibitor-1 in non-obese and obese individuals. Diabetologia 1998; 41: 65–71.
Belalcazar LM, Ballantyne CM, Lang W, Haffner SM, Rushing J, Schwenke DC et al. Metabolic factors, adipose tissue, and plasminogen activator inhibitor-1 levels in type 2 diabetes: findings from the look AHEAD study. Arterioscler Thromb Vasc Biol 2011; 31: 1689–1695.
Shoelson SE, Lee J, Goldfine AB . Inflammation and insulin resistance. J Clin Invest 2006; 116: 1793–1801.
Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S . Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007; 56: 1010–1013.
Trayhurn P . Adipocyte biology. Obes Rev 2007; 8 (Suppl 1): 41–44.
He G, Pedersen SB, Bruun JM, Lihn AS, Jensen PF, Richelsen B . Differences in plasminogen activator inhibitor 1 in subcutaneous versus omental adipose tissue in non-obese and obese subjects. Horm Metab Res 2003; 35: 178–182.
Samad F, Pandey M, Loskutoff DJ . Tissue factor gene expression in the adipose tissues of obese mice. Proc Natl Acad Sci USA 1998; 95: 7591–7596.
Hori Y, Gabazza EC, Yano Y, Katsuki A, Suzuki K, Adachi Y et al. Insulin resistance is associated with increased circulating level of thrombin-activatable fibrinolysis inhibitor in type 2 diabetic patients. J Clin Endocrinol Metab 2002; 87: 660–665.
Hori Y, Nakatani K, Morioka K, Katsuki A, Gabazza EC, Yano Y et al. Insulin enhanced thrombin-activable fibrinolysis inhibitor expression through PI3 kinase/Akt pathway. Int J Mol Med 2005; 15: 265–268.
Elbatarny HS, Netherton SJ, Ovens JD, Ferguson AV, Maurice DH . Adiponectin, ghrelin, and leptin differentially influence human platelet and human vascular endothelial cell functions: implication in obesity-associated cardiovascular diseases. Eur J Pharmacol 2007; 558: 7–13.
Konstantinides S, Schafer K, Koschnick S, Loskutoff DJ . Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest 2001; 108: 1533–1540.
Newell FS, Su H, Tornqvist H, Whitehead JP, Prins JB, Hutley LJ . Characterization of the transcriptional and functional effects of fibroblast growth factor-1 on human preadipocyte differentiation. FASEB J 2006; 20: 2615–2617.
Wabitsch M, Brenner RE, Melzner I, Braun M, Moller P, Heinze E et al. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord 2001; 25: 8–15.
Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS, Gimbrone MA . Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci USA 1986; 83: 4533–4537.
Neumann FJ, Ott I, Marx N, Luther T, Kenngott S, Gawaz M et al. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol 1997; 17: 3399–3405.
Zhou L, Sell H, Eckardt K, Yang Z, Eckel J . Conditioned medium obtained from in vitro differentiated adipocytes and resistin induce insulin resistance in human hepatocytes. FEBS Lett 2007; 581: 4303–4308.
Clement S, Juge-Aubry C, Sgroi A, Conzelmann S, Pazienza V, Pittet-Cuenod B et al. Monocyte chemoattractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatology 2008; 48: 799–807.
Wang Z, Lv J, Zhang R, Zhu Y, Zhu D, Sun Y et al. Co-culture with fat cells induces cellular insulin resistance in primary hepatocytes. Biochem Biophys Res Commun 2006; 345: 976–983.
Kaptoge S, White IR, Thompson SG, Wood AM, Lewington S, Lowe GD et al. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154 211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am J Epidemiol 2007; 166: 867–879.
Kopp CW, Kopp HP, Steiner S, Kriwanek S, Krzyzanowska K, Bartok A et al. Weight loss reduces tissue factor in morbidly obese patients. Obes Res 2003; 11: 950–956.
Cigolini M, Targher G, Bergamo AI, Tonoli M, Agostino G, De SG . Visceral fat accumulation and its relation to plasma hemostatic factors in healthy men. Arterioscler Thromb Vasc Biol 1996; 16: 368–374.
Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity 2008; 16: 1394–1399.
Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE . Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008; 103: 1372–1379.
van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008; 48: 449–457.
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–461.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Cartier A, Lemieux I, Almeras N, Tremblay A, Bergeron J, Despres JP . Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab 2008; 93: 1931–1938.
Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R et al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett 1989; 242: 237–239.
Faber DR, van der GY, Westerink J, Visseren FL . Increased visceral adipose tissue mass is associated with increased C-reactive protein in patients with manifest vascular diseases. Atherosclerosis 2010; 212: 274–280.
Mertens I, Van Gaal LF . Visceral fat as a determinant of fibrinolysis and hemostasis. Semin Vasc Med 2005; 5: 48–55.
Seki T, Healy AM, Fletcher DS, Noguchi T, Gelehrter TD . IL-1beta mediates induction of hepatic type 1 plasminogen activator inhibitor in response to local tissue injury. Am J Physiol 1999; 277: G801–G809.
Birgel M, Gottschling-Zeller H, Rohrig K, Hauner H . Role of cytokines in the regulation of plasminogen activator inhibitor-1 expression and secretion in newly differentiated subcutaneous human adipocytes. Arterioscler Thromb Vasc Biol 2000; 20: 1682–1687.
Zhang HH, Kumar S, Barnett AH, Eggo MC . Dexamethasone inhibits tumor necrosis factor-alpha-induced apoptosis and interleukin-1 beta release in human subcutaneous adipocytes and preadipocytes. J Clin Endocrinol Metab 2001; 86: 2817–2825.
Acknowledgements
This work was supported by a grant from the Leatare Foundation, Monaco and the Catharijne Foundation, the Netherlands.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Faber, D., Kalkhoven, E., Westerink, J. et al. Conditioned media from (pre)adipocytes stimulate fibrinogen and PAI-1 production by HepG2 hepatoma cells. Nutr & Diabetes 2, e52 (2012). https://doi.org/10.1038/nutd.2012.25
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nutd.2012.25
Keywords
This article is cited by
-
20 Years with SGBS cells - a versatile in vitro model of human adipocyte biology
International Journal of Obesity (2022)
-
The EGF epidermal growth factor counteracts Tat modulation of human endogenous retroviruses of the W family in astrocytes
Journal of NeuroVirology (2017)