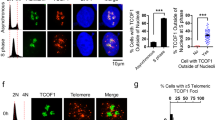
Abstract
Telomere end-binding proteins (TEBPs) bind to the guanine-rich overhang (G-overhang) of telomeres. Although the DNA binding properties of TEBPs have been investigated in vitro, little is known about their functions in vivo. Here we use RNA interference to explore in vivo functions of two ciliate TEBPs, TEBPα and TEBPβ. Silencing the expression of genes encoding both TEBPs shows that they cooperate to control the formation of an antiparallel guanine quadruplex (G-quadruplex) DNA structure at telomeres in vivo. This function seems to depend on the role of TEBPα in attaching telomeres in the nucleus and in recruiting TEBPβ to these sites. In vitro DNA binding and footprinting studies confirm the in vivo observations and highlight the role of the C terminus of TEBPβ in G-quadruplex formation. We have also found that G-quadruplex formation in vivo is regulated by the cell cycle–dependent phosphorylation of TEBPβ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Zakian, V.A. Telomeres: beginning to understand the end. Science 270, 1601–1607 (1995).
De Lange, T. Protection of mammalian telomeres. Oncogene 21, 532–540 (2002).
Jonsson, F. & Lipps, H.J. The biology of telomeres in hypotrichous ciliates. in Telomerases, Telomeres and Cancer (eds. Krupp, G. & Parwaresch, R.) 205–222 (Landes Bioscience, Kluwer Academic/Plenum Publishers, Georgetown, Texas, USA, 2002).
Klobutcher, L.A., Swanton, M.T., Donini, P. & Prescott, D.M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3′ terminus. Proc. Natl. Acad. Sci. USA 78, 3015–3019 (1981).
Wellinger, R.J., Ethier, K., Labrecque, P. & Zakian, V.A. Evidence for a new step in telomere maintenance. Cell 85, 423–433 (1996).
Makarov, V.L., Hirose, Y. & Langmore, J.P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88, 657–666 (1997).
Murzin, A.G. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 12, 861–867 (1993).
Mitton-Fry, R.M., Anderson, E.M., Hughes, T.R., Lundblad, V. & Wuttke, D.S. Conserved structure for single-stranded telomeric DNA recognition. Science 296, 145–147 (2002).
Theobald, D.L. & Wuttke, D.S. Prediction of multiple tandem OB-fold domains in telomere end-binding proteins Pot1 and Cdc13. Structure (Camb) 12, 1877–1879 (2004).
Gottschling, D.E. & Zakian, V.A. Telomere proteins: specific recognition and protection of the natural termini of Oxytricha macronuclear DNA. Cell 47, 195–205 (1986).
Peersen, O.B., Ruggles, J.A. & Schultz, S.C. Dimeric structure of the Oxytricha nova telomere end-binding protein alpha-subunit bound to ssDNA. Nat. Struct. Biol. 9, 182–187 (2002).
Gray, J.T., Celander, D.W., Price, C.M. & Cech, T.R. Cloning and expression of genes for the Oxytricha telomere-binding protein: specific subunit interactions in the telomeric complex. Cell 67, 807–814 (1991).
Fang, G. & Cech, T.R. Oxytricha telomere-binding protein: DNA-dependent dimerization of the alpha and beta subunits. Proc. Natl. Acad. Sci. USA 90, 6056–6060 (1993).
Horvath, M.P., Schweiker, V.L., Bevilacqua, J.M., Ruggles, J.A. & Schultz, S.C. Crystal structure of the Oxytricha nova telomere end binding protein complexed with single strand DNA. Cell 95, 963–974 (1998).
Fang, G. & Cech, T.R. The beta subunit of Oxytricha telomere-binding protein promotes G-quartet formation by telomeric DNA. Cell 74, 875–885 (1993).
Sundquist, W.I. & Klug, A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature 342, 825–829 (1989).
Williamson, J.R., Raghuraman, M.K. & Cech, T.R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell 59, 871–880 (1989).
Lipps, H.J. In vitro aggregation of the gene-sized DNA molecules of the ciliate Stylonychia mytilus. Proc. Natl. Acad. Sci. USA 77, 4104–4107 (1980).
Giraldo, R. & Rhodes, D. The yeast telomere-binding protein RAP1 binds to and promotes the formation of DNA quadruplexes in telomeric DNA. EMBO J. 13, 2411–2420 (1994).
Zahler, A.M., Williamson, J.R., Cech, T.R. & Prescott, D.M. Inhibition of telomerase by G-quartet DNA structures. Nature 350, 718–720 (1991).
Schaffitzel, C. et al. In vitro generated antibodies specific for telomeric guanine-quadruplex DNA react with Stylonychia lemnae macronuclei. Proc. Natl. Acad. Sci. USA 98, 8572–8577 (2001).
Meyer, G.F. & Lipps, H.J. The formation of polytene chromosomes during macronuclear development of the hypotrichous ciliate Stylonychia mytilus. Chromosoma 82, 309–314 (1981).
Murti, K.G. & Prescott, D.M. Topological organization of DNA molecules in the macronucleus of hypotrichous ciliated protozoa. Chromosome Res. 10, 165–173 (2002).
Lipps, H.J., Gruissem, W. & Prescott, D.M. Higher order DNA structure in macronuclear chromatin of the hypotrichous ciliate Oxytricha nova. Proc. Natl. Acad. Sci. USA 79, 2495–2499 (1982).
Postberg, J. et al. Association of the telomere-telomere binding protein-complex of hypotrichous ciliates with the nuclear matrix and dissociation during replication. J. Cell Sci. 114, 1861–1866 (2001).
de Lange, T. Human telomeres are attached to the nuclear matrix. EMBO J. 11, 717–724 (1992).
de Lara, J., Wydner, K.L., Hyland, K.M. & Ward, W.S. Fluorescent in situ hybridization of the telomere repeat sequence in hamster sperm nuclear structures. J. Cell. Biochem. 53, 213–221 (1993).
Laroche, T., Martin, S.G., Tsai-Pflugfelder, M. & Gasser, S.M. The dynamics of yeast telomeres and silencing proteins through the cell cycle. J. Struct. Biol. 129, 159–174 (2000).
Jonsson, F., Postberg, J., Schaffitzel, C. & Lipps, H.J. Organization of the macronuclear gene-sized pieces of stichotrichous ciliates into a higher order structure via telomere-matrix interactions. Chromosome Res. 10, 445–453 (2002).
Jackson, D.A., Yuan, J. & Cook, P.R. A gentle method for preparing cyto- and nucleo-skeletons and associated chromatin. J. Cell Sci. 90, 365–378 (1988).
Paschka, A.G. et al. The use of RNAi to analyze gene function in spirotrichous ciliates. Eur. J. Protistol. 39, 449–454 (2003).
Schaffitzel, C., Hanes, J., Jermutus, L. & Pluckthun, A. Ribosome display: an in vitro method for selection and evolution of antibodies from libraries. J. Immunol. Methods 231, 119–135 (1999).
Lipps, H.J. et al. Antibodies against Z DNA react with the macronucleus but not the micronucleus of the hypotrichous ciliate Stylonychia mytilus. Cell 32, 435–441 (1983).
Fang, G., Gray, J.T. & Cech, T.R. Oxytricha telomere-binding protein: separable DNA-binding and dimerization domains of the alpha-subunit. Genes Dev. 7, 870–882 (1993).
Fang, G. & Cech, T.R. Characterization of a G-quartet formation reaction promoted by the beta- subunit of the Oxytricha telomere-binding protein. Biochemistry 32, 11646–11657 (1993).
Horvath, M.P. & Schultz, S.C. DNA G-quartets in a 1.86 A resolution structure of an Oxytricha nova telomeric protein-DNA complex. J. Mol. Biol. 310, 367–377 (2001).
Froelich-Ammon, S.J., Dickinson, B.A., Bevilacqua, J.M., Schultz, S.C. & Cech, T.R. Modulation of telomerase activity by telomere DNA-binding proteins in Oxytricha. Genes Dev. 12, 1504–1514 (1998).
Hicke, B. et al. Phosphorylation of the Oxytricha telomere protein: possible cell cycle regulation. Nucleic Acids Res. 23, 1887–1893 (1995).
Obenauer, J.C., Cantley, L.C. & Yaffe, M.B. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 31, 3635–3641 (2003).
Juranek, S.A., Jönsson, F., Maercker, C. & Lipps, H.J. The telomeres of replicating macronuclear DNA molecules of the ciliate Stylonychia lemnae. Protistology 1, 148–151 (2000).
Kitagawa, M. et al. Butyrolactone I, a selective inhibitor of cdk2 and cdc2 kinase. Oncogene 8, 2425–2432 (1993).
Rhodes, D. & Giraldo, R. Telomere structure and function. Curr. Opin. Struct. Biol. 5, 311–322 (1995).
Riou, J.F. et al. Cell senescence and telomere shortening induced by a new series of specific G-quadruplex DNA ligands. Proc. Natl. Acad. Sci. USA 99, 2672–2677 (2002).
Rezler, E.M., Bearss, D.J. & Hurley, L.H. Telomeres and telomerases as drug targets. Curr. Opin. Pharmacol. 2, 415–423 (2002).
Ding, H. et al. Regulation of murine telomere length by Rtel: an essential gene encoding a helicase-like protein. Cell 117, 873–886 (2004).
Ammermann, D., Steinbruck, G., von Berger, L. & Hennig, W. The development of the macronucleus in the ciliated protozoan Stylonychia mytilus. Chromosoma 45, 401–429 (1974).
Timmons, L. & Fire, A. Specific interference by ingested dsRNA. Nature 395, 854 (1998).
Fairall, L., Chapman, L., Moss, H., de Lange, T. & Rhodes, D. Structure of the TRFH dimerization domain of the human telomeric proteins TRF and TRF2. Mol. Cell 8, 351–361 (2001).
Acknowledgements
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to H.J.L., a European Molecular Biology Organization short-term fellowship to K.P. and a Wenner-Gren Foundations fellowship to T.S. We thank T. Cech (University of Colorado, Boulder, Colorado, USA) for providing antibodies against the TEBP subunits and C. Schaffitzel (Swiss Federal Institute of Technology, Zurich) for providing antibodies against G-quadruplex DNA structures.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
A ClustalW sequence alignment of TEBPβ from Stylonychia lemnae and Oxytricha nova. (PDF 109 kb)
Supplementary Table 1
Stability of telomeric DNA upon RNAi mediated silencing of TEBPα or TEBPβ expression: no RNAi. (PDF 16 kb)
Supplementary Table 2
Stability of telomeric DNA upon RNAi mediated silencing of TEBPa or TEBPb expression: TEBPα RNAi. (PDF 16 kb)
Supplementary Table 3
Stability of telomeric DNA upon RNAi mediated silencing of TEBPa or TEBPb expression: TEBPβ RNAi. (PDF 16 kb)
Rights and permissions
About this article
Cite this article
Paeschke, K., Simonsson, T., Postberg, J. et al. Telomere end-binding proteins control the formation of G-quadruplex DNA structures in vivo. Nat Struct Mol Biol 12, 847–854 (2005). https://doi.org/10.1038/nsmb982
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb982
This article is cited by
-
Recognition and coacervation of G-quadruplexes by a multifunctional disordered region in RECQ4 helicase
Nature Communications (2023)
-
G-quadruplexes: a promising target for cancer therapy
Molecular Cancer (2021)
-
Telomerase subunit Est2 marks internal sites that are prone to accumulate DNA damage
BMC Biology (2021)
-
Single-molecule imaging reveals replication fork coupled formation of G-quadruplex structures hinders local replication stress signaling
Nature Communications (2021)
-
Conserved associations between G-quadruplex-forming DNA motifs and virulence gene families in malaria parasites
BMC Genomics (2020)