Abstract
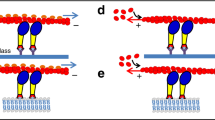
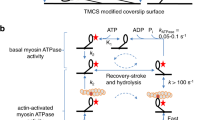
Myosin V is a calmodulin-binding motor protein. The dissociation of single calmodulin molecules from individual myosin V molecules at 1 μM Ca2+ correlates with a reduction in sliding velocity in an in vitro motility assay. The dissociation of two calmodulin molecules at 5 μM Ca2+ correlates with a detachment of actin filaments from myosin V. To mimic the regulation of myosin V motility by Ca2+ in a cell, caged Ca2+ coupled with a UV flash system was used to produce Ca2+ transients. During the Ca2+ transient, myosin V goes through the functional cycle of reduced sliding velocity, actin detachment and reattachment followed by the recovery of the sliding velocity. These results indicate that myosin V motility is regulated by Ca2+ through a reduction in actin-binding affinity resulting from the dissociation of single calmodulin molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ikura, M. Calcium binding and conformational response in EF-hand proteins. Trends Biochem. Sci. 21, 14–17 (1996).
Chin, D. & Means, A.R. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 10, 322–328 (2000).
Prekeris, R. & Terrian, D.M. Brain myosin V is a synaptic vesicle–associated motor protein: evidence for a Ca2+-dependent interaction with the synaptobrevin–synaptophysin complex. J. Cell Biol. 137, 1589–1601 (1997).
Evans, L.L., Lee, A.J., Bridgman, P.C. & Mooseker, M.S. Vesicle-associated brain myosin V can be activated to catalyze actin-based transport. J. Cell Sci. 111, 2055–2066 (1998).
Tabb, J.S., Molyneaux, B.J., Cohen, D.L., Kuznetsov, S.A. & Langford, G.M. Transport of ER vesicles on actin filaments in neurons by myosin V. J. Cell Sci. 111, 3221–3234 (1998).
Provance, D.W. Jr., Wei, M., Ipe, V. & Mercer, J.A. Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution. Proc. Natl. Acad. Sci. USA 93, 14554–14558 (1996).
Wu, X., Bowers, B., Wei, Q., Kocher, B. & Hammer, J.A. 3rd. Myosin V associates with melanosomes in mouse melanocytes: evidence that myosin V is an organelle motor. J. Cell Sci. 110, 847–859 (1997).
Karcher, R.L. et al. Cell cycle regulation of myosin V by calcium/calmodulin-dependent protein kinase II. Science 293, 1317–1320 (2001).
Hill, K.L., Catlett, N.L. & Weisman, L.S. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J. Cell Biol. 135, 1535–1549 (1996).
Catlett, N.L. & Weisman, L.S. The terminal tail region of a yeast myosin V mediates its attachment to vacuole membranes and sites of polarized growth. Proc. Natl. Acad. Sci. USA 95, 14799–14804 (1998).
Mehta, A.D. et al. Myosin V is a processive actin-based motor. Nature 400, 590–593 (1999).
Uemura, S., Higuchi, H., Olivares, A.O., De La Cruz E.M. & Ishiwata, S. Mechanochemical coupling of 12- and 24-nm substeps in a single myosin V motor. Nat. Struct Mol. Biol. 11, 877–883 (2004).
Yildiz, A. et al. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science 300, 2061–2065 (2003).
Espindola, F.S. et al. Biochemical and immunological characterization of p190–calmodulin complex from vertebrate brain: a novel calmodulin binding myosin. J. Cell Biol. 118, 359–368 (1992).
Espreafico, E.M. et al. Primary structure and cellular localization of chicken brain myosin V (p190), an unconventional myosin with calmodulin light chains. J. Cell Biol. 119, 1541–1557 (1992).
Cheney, R.E. et al. Brain myosin V is a two-headed unconventional myosin with motor activity. Cell 75, 13–23 (1993).
Cameron, L.C. et al. Calcium-induced quenching of intrinsic fluorescence in brain myosin V is linked to dissociation of calmodulin light chains. Arch. Biochem. Biophys. 355, 35–42 (1998).
Nascimento, A.A., Cheney, R.E., Tauhata, S.B., Larson, R.E. & Mooseker, M.S. Enzymatic characterization and functional domain mapping of brain myosin V. J. Biol. Chem. 271, 17561–17569 (1996).
Trybus, K.M., Krementsova, E. & Freyzon, Y. Kinetic characterization of a monomeric unconventional myosin V construct. J. Biol. Chem. 274, 27448–27456 (1999).
Homma, K., Saito, J., Ikebe, R. & Ikebe, M. Ca2+-dependent regulation of the motor activity of myosin V. J. Biol. Chem. 275, 34766–34771 (2000).
Martin, S.R. & Bayley, P.M. Regulatory implications of a novel mode of interaction of calmodulin with a double IQ motif target sequence from murine dilute myosin V. Protein Sci. 11, 2909–2923 (2002).
Nguyen, V.T., Higuchi, H. & Kamio, Y. Controlling pore assembly of staphylococcal γ-haemolysin by low temperature and by disulphide bond formation in double-cysteine LukF mutants. Mol. Microbiol. 45, 1485–1498 (2002).
Krementsov, D.N., Krementsova, E.B. & Trybus, K.M. Myosin V: regulation by calcium, calmodulin, and the tail domain. J. Cell Biol. 164, 877–886 (2004).
Cheney, R.E. Purification and assay of myosin V. Methods Enzymol. 298, 3–18 (1998).
Poenie, M., Alderton, J., Tsien, R.Y. & Steinhardt, R.A. Changes of free calcium levels with stages of the cell division cycle. Nature 315, 147–149 (1985).
Sakamoto, T., Amitani, I. Yokota, E. & Ando, T. Direct observation of processive movement by individual myosin V molecules. Biochem. Biophys. Res. Commun. 272, 586–590 (2000).
Li, X.D., Mabuchi, K., Ikebe, R. & Ikebe, M. Ca2+-induced activation of ATPase activity of myosin Va is accompanied with a large conformational change. Biochem. Biophys. Res. Commun. 12, 538–545 (2004).
Tauhata, S.B., dos Santos, D.V., Taylor, E.W., Mooseker, M.S. & Larson, R.E. High affinity binding of brain myosin Va to F-actin induced by calcium in the presence of ATP. J. Biol. Chem. 276, 39812–39818 (2001).
Gomez, T.M., Robles E., Poo M. & Spitzer N.C. Filopodial calcium transients promote substrate-dependent growth cone turning. Science 291, 1983–1987 (2001).
Ellis-Davies, G.C. & Kaplan, J.H. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc. Natl. Acad. Sci. USA 91, 187–191 (1994).
Spudich, J.A. & Watt, S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 246, 4866–4871 (1971).
Horiuti, K. et al. Mechanism of action of 2,3-butanedione 2-monoxime on contraction of frog skeletal muscle fibres. J. Muscle. Res. Cell Motil. 9, 156–164 (1988).
Nguyen, V.T., Kamio, Y. & Higuchi, H. Single molecule imaging of cooperative assembly of γ-hemolysin on erythrocyte membranes. EMBO J. 22, 4968–4979 (2003).
Funatsu, T., Harada, Y., Tokunaga, M., Saito, K. & Yanagida, T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature 374, 555–559 (1995).
Dantzig, J.A., Higuchi, H. & Goldman, Y.E. Studies of molecular motors using caged compounds. Methods Enzymol. 291, 307–348 (1998).
West, J.M., Higuchi, H., Ishijima, A. & Yanagida, T. Modification of the bi-directional sliding movement of actin filaments along native thick filaments isolated from a clam. J. Muscle Res. Cell Motil. 17, 637–646 (1996).
Acknowledgements
We thank N. Sasaki and S. Uemura for giving important suggestions and helping in purification of myosin V, and T. Ando and J. M. West for critical reading of the manuscript. The research was supported by Grants-in-Aid for Scientific Research in Priority Areas from the Japan Ministry of Education, Culture, Sports, Science and Technology (H.H.). H.A.N. was the recipient of a postdoctoral scholarship from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
The reversible attachment and movement of actin filaments by myosin–V. An in vitro motility was performed in the presence of 10 µM CaM and transient Ca2+concentrations, which was generated locally by UV photolysis of caged Ca2+ within a time frame of 4 s. The number indicates the time in seconds and tens of milliseconds. The time period of the UV flash was indicated by “UV”. Bar 10 µm. This movie is a supplement for Figure 4a. (MOV 1437 kb)
Rights and permissions
About this article
Cite this article
Nguyen, H., Higuchi, H. Motility of myosin V regulated by the dissociation of single calmodulin. Nat Struct Mol Biol 12, 127–132 (2005). https://doi.org/10.1038/nsmb894
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb894
This article is cited by
-
Expression of hypoxia-inducible genes is suppressed in altered gravity due to impaired nuclear HIF1α accumulation
Scientific Reports (2023)
-
Regulation of class V myosin
Cellular and Molecular Life Sciences (2018)
-
Walking to work: roles for class V myosins as cargo transporters
Nature Reviews Molecular Cell Biology (2012)
-
Dynamics of different-sized solid-state nanocrystals as tracers for a drug-delivery system in the interstitium of a human tumor xenograft
Breast Cancer Research (2009)
-
Expression of the Dominant-Negative Tail of Myosin Va Enhances Exocytosis of Large Dense Core Vesicles in Neurons
Cellular and Molecular Neurobiology (2009)