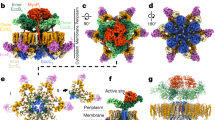
Abstract
The type III secretion system (TTSS) mediates the specific translocation of bacterial proteins into the cytoplasm of eukaryotic cells, a process essential for the virulence of many Gram-negative pathogens. The enteropathogenic Escherichia coli TTSS protein EspA forms a hollow extracellular filament believed to be a molecular conduit for type III protein translocation. Structural analysis of EspA has been hampered by its polymeric nature. We show that EspA alone is sufficient to form filamentous structures in the absence of other pathogenicity island–encoded proteins. CesA is the recently proposed chaperone of EspA, and we demonstrate that CesA traps EspA in a monomeric state and inhibits its polymerization. Crystallographic analysis of the heterodimeric CesA–EspA complex at a resolution of 2.8 Å reveals that EspA contains two long a-helices, which are involved in extensive coiled-coil interactions with CesA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Hueck, C.J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62, 379–433 (1998).
Galan, J.E. & Collmer, A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284, 1322–1328 (1999).
Stebbins, C.E. & Galan, J.E. Structural mimicry in bacterial virulence. Nature 412, 701–705 (2001).
Blocker, A. et al. Structure and composition of the Shigella flexneri 'needle complex,' a part of its type III secreton. Mol. Microbiol. 39, 652–663 (2001).
Kubori, T. et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280, 602–605 (1998).
Daniell, S.J. et al. The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell. Microbiol. 3, 865–871 (2001).
Aizawa, S.I. Bacterial flagella and type III secretion systems. FEMS Microbiol. Lett. 202, 157–164 (2001).
Cornelis, G.R. & Wolf-Watz, H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23, 861–867 (1997).
Vallance, B.A. & Finlay, B.B. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97, 8799–8806 (2000).
Knutton, S. et al. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17, 2166–2176 (1998).
Lai, L.C., Wainwright, L.A., Stone, K.D. & Donnenberg, M.S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect. Immun. 65, 2211–2217 (1997).
Sekiya, K. et al. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. USA 98, 11638–11643 (2001).
Ide, T. et al. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell. Microbiol. 3, 669–679 (2001).
Daniell, S.J. et al. 3D structure of EspA filaments from enteropathogenic Escherichia coli. Mol. Microbiol. 49, 301–308 (2003).
Creasey, E.A. et al. CesAB is an enteropathogenic Escherichia coli chaperone for the type-III translocator proteins EspA and EspB. Microbiology 149, 3639–3647 (2003).
Feldman, M.F. & Cornelis, G.R. The multitalented type III chaperones: all you can do with 15 kDa. FEMS Microbiol. Lett. 219, 151–158 (2003).
Parsot, C., Hamiaux, C. & Page, A.L. The various and varying roles of specific chaperones in type III secretion systems. Curr. Opin. Microbiol. 6, 7–14 (2003).
Lupas, A., Van Dyke, M. & Stock, J. Predicting coiled coils from protein sequences. Science 252, 1162–1164 (1991).
Roe, A.J. et al. Heterogeneous surface expression of EspA translocon filaments by Escherichia coli O157:H7 is controlled at the posttranscriptional level. Infect. Immun. 71, 5900–5909 (2003).
Luo, Y. et al. Structural and biochemical characterization of the type III secretion chaperones CesT and SigE. Nat. Struct. Biol. 8, 1031–1036 (2001).
Stebbins, C.E. & Galan, J.E. Maintenance of an unfolded polypeptide by a cognate chaperone in bacterial type III secretion. Nature 414, 77–81 (2001).
Birtalan, S.C., Phillips, R.M. & Ghosh, P. Three-dimensional secretion signals in chaperone–effector complexes of bacterial pathogens. Mol. Cell 9, 971–980 (2002).
Pallen, M.J., Dougan, G. & Frankel, G. Coiled coil domains in proteins secreted by type III secretion systems. Mol. Microbiol. 25, 423–425 (1997).
Delahay, R.M. & Frankel, G. Coiled coil proteins associated with type III secretion systems: a versatile domain revisited. Mol. Microbiol. 45, 905–916 (2002).
Zurawski, D.V. & Stein, M.A. The SPI2-encoded SseA chaperone has discrete domains required for SseB stabilization and export, and binds within the C-terminus of SseB and SseD. Microbiology 150, 2055–2068 (2004).
Auvray, F., Thomas, J., Fraser, G.M. & Hughes, C. Flagellin polymerisation control by a cytosolic export chaperone. J. Mol. Biol. 308, 221–229 (2001).
Evdokimov, A.G. et al. Similar modes of polypeptide recognition by export chaperones in flagellar biosynthesis and type III secretion. Nat. Struct. Biol. 10, 789–793 (2003).
Delahay, R.M. et al. The coiled coil domain of EspA is essential for the assembly of the type III secretion translocon on the surface of enteropathogenic Escherichia coli. J. Biol. Chem. 274, 35969–35974 (1999).
Samatey, F.A. et al. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 410, 331–337 (2001).
Yonekura, K., Maki-Yonekura, S. & Namba, K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 424, 643–650 (2003).
Namba, K., Yamashita, I. & Vonderviszt, F. Structure of the core and central channel of bacterial flagella. Nature 342, 648–654 (1989).
Cordes, F.S. et al. Helical structure of the needle of the type III secretion system of Shigella flexneri. J. Biol. Chem. 278, 17103–17107 (2003).
Leslie, A.G.W. Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4 and ESF-EACMB Newsletter on Protein Crystallography Vol. 26 (Daresbury Laboratory, Warrington, UK, 1992).
Evans, P.R. Data reduction. Proceedings of the CCP4 Study Weekend on Data Collection & Processing (eds. Sawyer, L., Isaacs, N. & Bailey, S.) 114–122 (Daresbury Laboratory, Warrington, UK, 1993).
Terwilliger, T.C. & Berendzen, J. Automated MAD and MIR structure solution. Acta Crystallogr. D 55, 849–861 (1999).
Terwilliger, T.C. Maximum-likelihood density modification. Acta Crystallogr. D 56, 965–972 (2000).
McRee, D.E. XtalView/Xfit—a versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 125, 156–165 (1999).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Merritt, E.A.B. & Bacon, D.J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997).
Acknowledgements
We thank A.L. Lovering, M.G. Bertero, D. Lim, and P.I. Lario for assistance in data collection and analyses, R.A. Pfuetzner for assistance in multiangle light scattering, S. He for mass spectrometry service, G. Martens and E. Humphries for assistance in transmission EM, N.A. Thomas and W. Deng for advice and support and F. Rosell for assistance in CD spectroscopy. We thank the US Department of Energy and the staff at the Advanced Light Source beamline 8.2.2 for access and assistance in data collection. C.K. Yip is supported by training awards from the Natural Sciences and Engineering Research Council of Canada and the Michael Smith Foundation for Health Research. N.C.J. Strynadka thanks the Canadian Institutes of Health Research, Howard Hughes Medical Institute and Canadian Bacterial Diseases Network for financial support for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Electron density map. (PDF 83 kb)
Rights and permissions
About this article
Cite this article
Yip, C., Finlay, B. & Strynadka, N. Structural characterization of a type III secretion system filament protein in complex with its chaperone. Nat Struct Mol Biol 12, 75–81 (2005). https://doi.org/10.1038/nsmb879
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb879
This article is cited by
-
Identification and analysis of structurally critical fragments in HopS2
BMC Bioinformatics (2019)
-
Assembly, structure, function and regulation of type III secretion systems
Nature Reviews Microbiology (2017)
-
The SseC translocon component in Salmonella enterica serovar Typhimurium is chaperoned by SscA
BMC Microbiology (2013)
-
Role of EscU auto-cleavage in promoting type III effector translocation into host cells by enteropathogenic Escherichia coli
BMC Microbiology (2011)
-
Using nanoelectrospray ion mobility spectrometry (GEMMA) to determine the size and relative molecular mass of proteins and protein assemblies: a comparison with MALLS and QELS
Analytical and Bioanalytical Chemistry (2011)