Abstract
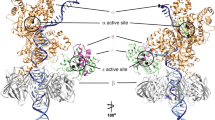
Pol λ is a family X member believed to fill short gaps during DNA repair. Here we report crystal structures of Pol λ representing three steps in filling a single-nucleotide gap. These structures indicate that, unlike other DNA polymerases, Pol λ does not undergo large subdomain movements during catalysis, and they provide a clear characterization of the geometry and stereochemistry of the in-line nucleotidyl transfer reaction.
This is a preview of subscription content, access via your institution
Access options


Similar content being viewed by others
References
Bebenek, K. & Kunkel, T.A. Functions of DNA polymerases. In DNA Repair and Replication, Advances in Protein Chemistry Vol. 69 (ed. Yang, W.) 137–165 (Elsevier, San Diego, 2004).
Sawaya, M.R., et al. Biochemistry 36, 11205–11215 (1997).
Doublie, S. & Ellenberger, T. Curr. Opin. Struct. Biol. 8, 704–712 (1998).
Johnson, S.J. et al. Proc. Natl. Acad. Sci. USA 100, 3895–3900 (2003).
Li, Y., et al. EMBO J. 17, 7514–7525 (1998).
Kunkel, T.A. & Bebenek, K. Annu. Rev. Biochem. 69, 497–529 (2000).
Garcia-Diaz, M. et al. J. Mol. Biol. 301, 851–867 (2000).
Lee, J.W. et al. J. Biol. Chem. 279, 805–811 (2004).
Garcia-Diaz, M. et al. J. Biol. Chem. 276, 34659–34663 (2001).
Garcia-Diaz, M. et al. Mol. Cell 13, 561–572 (2004).
Osheroff, W.P. et al. J. Biol. Chem. 275, 28033–28038 (2000).
Brautigam, C.A. & Steitz, T.A. Curr. Opin. Struct. Biol. 8, 54–63 (1998).
Burgers, P.M. & Eckstein, F. Biochemistry 18, 450–454 (1979).
Acknowledgements
The authors thank W. Beard, L. Pedersen and J. Boyington for critical review of the manuscript. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. W-31-109-Eng-38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Superimposition of Pol γ complexes (PDF 221 kb)
Supplementary Fig. 2
Minor groove interactions in the active site. (PDF 81 kb)
Supplementary Fig. 3
Coordination of the ddTTP with the catalytic metal. (PDF 125 kb)
Supplementary Table 1
Crystallographic data statistics. (PDF 55 kb)
Rights and permissions
About this article
Cite this article
Garcia-Diaz, M., Bebenek, K., Krahn, J. et al. A closed conformation for the Pol λ catalytic cycle. Nat Struct Mol Biol 12, 97–98 (2005). https://doi.org/10.1038/nsmb876
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb876
This article is cited by
-
Analysis of diverse double-strand break synapsis with Polλ reveals basis for unique substrate specificity in nonhomologous end-joining
Nature Communications (2022)
-
Watching right and wrong nucleotide insertion captures hidden polymerase fidelity checkpoints
Nature Communications (2022)
-
Watching a double strand break repair polymerase insert a pro-mutagenic oxidized nucleotide
Nature Communications (2021)
-
Structural snapshots of human DNA polymerase μ engaged on a DNA double-strand break
Nature Communications (2020)
-
Insights into DNA polymerase δ’s mechanism for accurate DNA replication
Journal of Molecular Modeling (2019)