Abstract
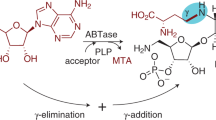
The flavoenzyme uridine 5′-diphosphate (UDP)-galactopyranose mutase (UGM) catalyzes the interconversion of UDP-galactopyranose (UDP-Galp) and UDP-galactofuranose (UDP-Galf). The latter is an essential precursor to the cell wall arabinogalactan of Mycobacterium tuberculosis. The catalytic mechanism for this enzyme had not been elucidated. Here, we provide evidence for a mechanism in which the flavin cofactor assumes a new role. Specifically, the N5 of the reduced anionic flavin cofactor captures the anomeric position of the galactose residue with release of UDP. Interconversion of the isomers occurs via a flavin-derived iminium ion. To trap this putative intermediate, we treated UGM with radiolabeled UDP-Galp and sodium cyanoborohydride; a radiolabeled flavin-galactose adduct was obtained. Ultraviolet-visible spectroscopy and mass spectrometry indicate that this product is an N5-alkyl flavin. We anticipate that the clarification of the catalytic mechanism for UGM will facilitate the development of anti-mycobacterial agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bloom, B.R. & Murray, C.J. Tuberculosis: commentary on a reemergent killer. Science 257, 1055–1064 (1992).
World Health Organization. Fact Sheet No. 104 (World Health Organization, Geneva, 2002).
Brennan, P. & Nikaido, H. The envelope of Mycobacteria. Annu. Rev. Biochem. 64, 29–63 (1995).
Houseknecht, J.B. & Lowary, T.L. Chemistry and biology of arabinofuranosyl- and galactofuranosyl-containing polysaccharides. Curr. Opin. Chem. Biol. 5, 677–682 (2001).
Lowary, T.L. D-arabinofuranosides from Mycobacteria: synthesis and conformation (reprinted from Glycochemistry: Principles, Synthesis, and Applications (eds. Wang, P.G. & Bertozzi, C.R., Marcel Dekker, New York) 133–162, 2001), J. Carbohydr. Chem. 21, 691–722 (2002).
Weston, A. et al. Biosynthetic origin of mycobacterial cell wall galactofuranosyl residues. Tuber. Lung Dis. 78, 123–131 (1997).
Pan, F., Jackson, M., Ma, Y.F. & McNeil, M. Cell wall core galactofuran synthesis is essential for growth of Mycobacteria. J. Bacteriol. 183, 3991–3998 (2001).
Pedersen, L.L. & Turco, S.J. Galactofuranose metabolism: a potential target for antimicrobial chemotherapy. Cell. Mol. Life Sci., 60, 259–266 (2003).
Barlow, J.N., Girvin, M.E. & Blanchard, J.S. Positional isotope exchange catalyzed by UDP-galactopyranose mutase. J. Am. Chem. Soc. 121, 6968–6969 (1999).
Zhang, Q. & Liu, H. Studies of UDP-galactopyranose mutase from Escherichia coli: an unusual role for reduced FAD in its catalysis. J. Am. Chem. Soc. 122, 9065–9070 (2000).
Stevenson, G. et al. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J. Bacteriol. 176, 4144–4156 (1994).
Massey, V. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans. 28, 283–296 (2000).
Ghisla, S. & Massey, V. Mechanisms of flavoprotein-catalyzed reactions. Eur. J. Biochem. 181, 1–17 (1989).
Sanders, D.A.R. et al. UDP-galactopyranose mutase has a novel structure and mechanism. Nat. Struct. Biol. 8, 858–863 (2001).
Klopprogge, K. & Schmitz, R.A. NifL of Klebsiella pneumoniae: redox characterization in relation to the nitrogen source. Biochim. Biophys. Acta. 1431, 462–470 (1999).
Chang, Y.Y. & Cronan, J.E. Common ancestry of Escherichia coli pyruvate oxidase and the acetohydroxy acid synthases of the branched-chain amino acid biosynthetic pathway. J. Bacteriol. 170, 3937–3945 (1988).
Chipman, D., Barak, Z. & Schloss, J.V. Biosynthesis of 2-aceto-2-hydroxy acids: acetolactate synthases and acetohydroxyacid synthases. Biochim. Biophys. Acta 1385, 401–419 (1998).
Huang, Z., Zhang, Q. & Liu, H. Reconstitution of UDP-galactopyranose mutase with 1-deaza-FAD and 5-deaza-FAD: analysis and mechanistic implications. Bioorg. Chem. 31, 494–502 (2003).
Barlow, J.N. & Blanchard, J.S. Enzymatic synthesis of UDP-(3-deoxy-3-fluoro)-D-galactose and UDP-(2-deoxy-2-fluoro)-D-galactose and substrate activity with UDP-galactopyranose mutase. Carbohydr. Res. 328, 473–480 (2000).
Fullerton, S.W.B et al. Potentiometric analysis of UDP-galactopyranose mutase: stabilization of the flavosemiquinone by substrate. Biochemistry 42, 2104–2109 (2003).
Schwarz, H.A. & Dodson, R.W. Reduction potentials of CO2− and the alcohol radicals. J. Phys. Chem. 93, 409–414 (1989).
Fritz, G. et al. Structure of adenylylsulfate reductase from the hyperthermophilic Archaeoglobus fulgidus at 1.6-Å resolution. Proc. Natl. Acad. Sci. USA 99, 1836–1841 (2002).
Ghisla, S. & Massey, V. New flavins for old: artificial flavins as active site probes of flavoproteins. Biochem. J. 239, 1–12 (1986).
Williams, R.F. & Bruice, T.C. The kinetics and mechanism of 1,5-dihydroflavin reduction of carbonyl compounds and flavin oxidation of alcohols. J. Am. Chem. Soc. 98, 7752–7768 (1976).
Kemal, C. & Bruice, T.C. The chemistry of an N5-methyl-1,5-dihydroflavin and its aminium cation radical. J. Am. Chem. Soc. 98, 3955–3964 (1976).
Hemmerich, P., Ghisla, S., Hartman, U. & Muller, F. In Flavins and Flavoproteins (ed. Kamin, H.) 83–105 (University Park Press, Durham, North Carolina, USA, 1969).
Porter, D.J.T., Voet, J.G. & Bright, H.J. Direct evidence for carbanions and covalent N5-flavine-carbanion adducts as catalytic intermediates in the oxidation of nitroethane by D-amino acid oxidase. J. Biol. Chem. 248, 4400–4416 (1973).
Williams, R.F., Shinkai, S.S. & Bruice, T.C. Kinetics and mechanisms of the 1,5-dihydroflavin reduction of carbonyl compounds and the flavin oxidation of alcohols. J. Am. Chem. Soc. 99, 921–931 (1977).
Edwards, J.O. & Pearson, R.G. The factors determining nucleophilic reactivities. J. Am. Chem. Soc. 84, 16–24 (1962).
Hoz, S. & Buncel, E. The α-effect—a critical examination of the phenomenon and its origin. Israel J. Chem. 26, 313–319 (1985).
Lee, Y.T. & Fisher, J.F. A mechanistic study of the dihydroflavin reductive cleavage of the dihydroflavin-tetrahydronaphthalene epoxide adducts. Bioorg. Chem. 28, 163–175 (2000).
Kallen, R.G. & Jencks, W.P. The mechanism of the condensation of formaldehyde with tetrahydrofolic acid. J. Biol. Chem. 241, 5851–5863 (1966).
Matthews, R.G. Are the redox properties of tetrahydrofolate cofactors utilized in folate-dependent reactions? Fed. Proc. 41, 2600–2604 (1982).
Walsh, C. Flavin coenzymes: at the crossroads of biological redox chemistry. Acc. Chem. Res. 13, 148–155 (1980).
Fitzpatrick, P.F. Substrate dehydrogenation by flavoproteins. Acc. Chem. Res. 34, 299–307 (2001).
Marlow, A.L. & Kiessling, L.L. Improved chemical synthesis of UDP-galactofuranose. Org. Lett. 3, 2517–2519 (2001).
Acknowledgements
We acknowledge R. Raines, P. Ludden (University of Wisconsin Madison) and the W. M. Keck Center for use of equipment. We thank R. Raines, A.Eschenmoser, W. Cleland, G. Reed, M. Wolfe, M. Vestling, and R. Derda for helpful conversations. This research was supported by the US National Science Foundation (CHE9357093), the US National Institutes of Health (NIH) (GM49975) and the Mizutani Foundation for Glycoscience. M.S.H., E.E.C. and T.D.G. were supported by the NIH Biotechnology Training Program (GM 08349). M.S.H. was supported by an NIH predoctoral fellowship (GM 18750).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Soltero-Higgin, M., Carlson, E., Gruber, T. et al. A unique catalytic mechanism for UDP-galactopyranose mutase. Nat Struct Mol Biol 11, 539–543 (2004). https://doi.org/10.1038/nsmb772
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb772
This article is cited by
-
A new role for an old cofactor
Nature Structural & Molecular Biology (2004)