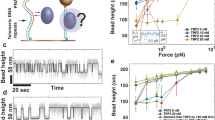
Abstract
Telomeres can fold into t-loops that may result from the invasion of the 3′ overhang into duplex DNA. Their formation is facilitated in vitro by the telomeric protein TRF2, but very little is known regarding the mechanisms involved. Here we reveal that TRF2 generates positive supercoiling and condenses DNA. Using a variety of TRF2 mutants, we demonstrate a strong correlation between this topological activity and the ability to stimulate strand invasion. We also report that these properties require the combination of the TRF-homology (TRFH) domain of TRF2 with either its N- or C-terminal DNA-binding domains. We propose that TRF2 complexes, by constraining DNA around themselves in a right-handed conformation, can induce untwisting of the neighboring DNA, thereby favoring strand invasion. Implications of this topological model in t-loop formation and telomere homeostasis are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chan, S.R. & Blackburn, E.H. Telomeres and telomerase. Phil. Trans. R. Soc. Lond. B 359, 109–121 (2004).
Smogorzewska, A. & De Lange, T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 73, 177–208 (2004).
Wenz, C. et al. Human telomerase contains two cooperating telomerase RNA molecules. EMBO J. 20, 3526–3534 (2001).
Wang, Y. & Patel, D.J. Guanine residues in d(T2AG3) and d(T2G4) form parallel-stranded potassium cation stabilized G-quadruplexes with anti glycosidic torsion angles in solution. Biochemistry 31, 8112–8119 (1992).
Griffith, J.D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 (1999).
Stansel, R.M., de Lange, T. & Griffith, J.D. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 20, 5532–5540 (2001).
Ancelin, K. et al. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol. Cell. Biol. 22, 3474–3487 (2002).
Wang, R.C., Smogorzewska, A. & de Lange, T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119, 355–368 (2004).
Dunham, M.A., Neumann, A.A., Fasching, C.L. & Reddel, R.R. Telomere maintenance by recombination in human cells. Nat. Genet. 26, 447–450 (2000).
Bauman, P. & Cech, T.R. Pot1, the putative telomere end binding protein in fission yeast and humans. Science 292, 1171–1175 (2001).
Chong, L. et al. A human telomeric protein. Science 270, 1663–1667 (1995).
Bilaud, T. et al. Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 17, 236–239 (1997).
Broccoli, D., Smogorzewska, A., Chong, L. & de Lange, T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 17, 231–235 (1997).
Holloman, W.K., Wiegand, R., Hoessli, C. & Radding, C.M. Uptake of homologous single-stranded fragments by superhelical DNA: a possible mechanism for initiation of genetic recombination. Proc. Natl. Acad. Sci. USA 72, 2394–2398 (1975).
Voloshin, O.N. et al. An eclectic DNA structure adopted by human telomeric sequence under superhelical stress and low pH. J. Biomol. Struct. Dyn. 9, 643–652 (1992).
Henderson, E. et al. Structure, synthesis, and regulation of telomeres. Cancer Cells 6, 453–461 (1988).
Okabe, J., Eguchi, A., Masago, A., Hayakawa, T. & Nakanishi, M. TRF1 is a critical trans-acting factor required for de novo telomere formation in human cells. Hum. Mol. Genet. 9, 2639–2650 (2000).
Cox, M.M. The bacterial RecA protein as a motor protein. Annu. Rev. Microbiol. 57, 551–577 (2003).
Verdun, R.E. & Karlseder, J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell 127, 709–720 (2006).
Court, R., Chapman, L., Fairall, L. & Rhodes, D. How the human telomeric proteins TRF1 and TRF2 recognize telomeric DNA: a view from high-resolution crystal structures. EMBO Rep. 6, 39–45 (2005).
Fouche, N. et al. The basic domain of TRF2 directs binding to DNA junctions irrespective of the presence of TTAGGG repeats. J. Biol. Chem. 281, 37486–37495 (2006).
Dantzer, F. et al. Functional interaction between poly(ADP-Ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2. Mol. Cell. Biol. 24, 1595–1607 (2004).
Li, B., Oestreich, S. & de Lange, T. Identification of human Rap1: implications for telomere evolution. Cell 101, 471–483 (2000).
Opresko, P.L. et al. Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. J. Biol. Chem. 277, 41110–41119 (2002).
Shure, M., Pulleyblank, D.E. & Vinograd, J. The problems of eukaryotic and prokaryotic DNA packaging and in vivo conformation posed by superhelix density heterogeneity. Nucleic Acids Res. 4, 1183–1205 (1977).
Waldmann, T., Eckerich, C., Baack, M. & Gruss, C. The ubiquitous chromatin protein DEK alters the structure of DNA by introducing positive supercoils. J. Biol. Chem. 277, 24988–24994 (2002).
Musgrave, D.R., Sandman, K.M. & Reeve, J.N. DNA binding by the archaeal histone HMf results in positive supercoiling. Proc. Natl. Acad. Sci. USA 88, 10397–10401 (1991).
Klungsoyr, H.K. & Skarstad, K. Positive supercoiling is generated in the presence of Escherichia coli SeqA protein. Mol. Microbiol. 54, 123–131 (2004).
Rau, D.C., Gellert, M., Thoma, F. & Maxwell, A. Structure of the DNA gyrase-DNA complex as revealed by transient electric dichroism. J. Mol. Biol. 193, 555–569 (1987).
Yoshimura, S.H., Maruyama, H., Ishikawa, F., Ohki, R. & Takeyasu, K. Molecular mechanisms of DNA end-loop formation by TRF2. Genes Cells 9, 205–218 (2004).
Rivetti, C., Guthold, M. & Bustamante, C. Wrapping of DNA around the E. coli RNA polymerase open promoter complex. EMBO J. 18, 4464–4475 (1999).
Shin, M. et al. DNA looping-mediated repression by histone-like protein H-NS: specific requirement of Eσ70 as a cofactor for looping. Genes Dev. 19, 2388–2398 (2005).
Belotserkovskii, B.P., Krasilnikova, M.M., Veselkov, A.G. & Frank-Kamenetskii, M.D. Kinetic trapping of H-DNA by oligonucleotide binding. Nucleic Acids Res. 20, 1903–1908 (1992).
Budarf, M. & Blackburn, E. S1 nuclease sensitivity of a double-stranded telomeric DNA sequence. Nucleic Acids Res. 15, 6273–6292 (1987).
Gilson, E., Muller, T., Sogo, J., Laroche, T. & Gasser, S.M. RAP1 stimulates single- to double-strand association of yeast telomeric DNA: implications for telomere-telomere interactions. Nucleic Acids Res. 22, 5310–5320 (1994).
Huertas, D., Lipps, H. & Azorin, F. Characterization of the structural conformation adopted by (TTAGGG)n telomeric DNA repeats of different length in closed circular DNA. J. Biomol. Struct. Dyn. 12, 79–90 (1994).
Lyamichev, V.I. et al. An unusual DNA structure detected in a telomeric sequence under superhelical stress and at low pH. Nature 339, 634–637 (1989).
Brunori, M. et al. TRF2 inhibition promotes anchorage-independent growth of telomerase-positive human fibroblasts. Oncogene 25, 990–997 (2006).
van Steensel, B., Smogorzewska, A. & de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 (1998).
Zhu, X.D., Kuster, B., Mann, M., Petrini, J.H. & de Lange, T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 25, 347–352 (2000).
Griffith, J., Bianchi, A. & de Lange, T. TRF1 promotes parallel pairing of telomeric tracts in vitro. J. Mol. Biol. 278, 79–88 (1998).
Miller, K.M. & Cooper, J.P. The telomere protein Taz1 is required to prevent and repair genomic DNA breaks. Mol. Cell 11, 303–313 (2003).
Kelleher, C., Kurth, I. & Lingner, J. Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro. Mol. Cell. Biol. 25, 808–818 (2005).
Henricksen, L.A., Umbricht, C.B. & Wold, M.S. Recombinant replication protein A: expression, complex formation, and functional characterization. J. Biol. Chem. 269, 11121–11132 (1994).
Angelov, D., Novakov, E., Khochbin, S. & Dimitrov, S. Ultraviolet laser footprinting of histone H1(0)-four-way junction DNA complexes. Biochemistry 38, 11333–11339 (1999).
Spassky, A. & Angelov, D. Influence of the local helical conformation on the guanine modifications generated from one-electron DNA oxidation. Biochemistry 36, 6571–6576 (1997).
Lyubchenko, Y.L., Oden, P.I., Lampner, D., Lindsay, S.M. & Dunker, K.A. Atomic force microscopy of DNA and bacteriophage in air, water and propanol: the role of adhesion forces. Nucleic Acids Res. 21, 1117–1123 (1993).
Acknowledgements
We thank R. Rahmouni for advice. This work was supported by grants from the Ligue Nationale contre le Cancer (“équipe labellisée”) and from “Région Centre”. C.L. is supported by fellowships from the Association pour la Recherche sur le Cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Characterization of the telomeric strand invasion reaction. (PDF 144 kb)
Supplementary Fig. 2
DNA-binding properties of the TRF2 mutant forms used in this study. (PDF 223 kb)
Supplementary Fig. 3
Characterization of the TRF2-mediated strand invasion reaction. (PDF 109 kb)
Supplementary Fig. 4
TRF2 does not behave as a RecA-type protein. (PDF 101 kb)
Supplementary Fig. 5
TRF2, TRF2ΔB and TRF2ΔM form DNA complexes that do not migrate on polyacrylamide gels. (PDF 68 kb)
Supplementary Fig. 6
Topoisomers obtained in the topological assay do not originate from contaminations in the protein preparation. (PDF 65 kb)
Supplementary Fig. 7
TRF1, TRF2DM, TRF2DB and TRF2 do not create the same amount of supercoiling. (PDF 37 kb)
Rights and permissions
About this article
Cite this article
Amiard, S., Doudeau, M., Pinte, S. et al. A topological mechanism for TRF2-enhanced strand invasion. Nat Struct Mol Biol 14, 147–154 (2007). https://doi.org/10.1038/nsmb1192
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1192
This article is cited by
-
Exploring TRF2-Dependent DNA Distortion Through Single-DNA Manipulation Studies
Communications Biology (2024)
-
Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization
Nature Reviews Molecular Cell Biology (2021)
-
RNA-DNA strand exchange by the Drosophila Polycomb complex PRC2
Nature Communications (2020)
-
Heterochromatin replication goes hand in hand with telomere protection
Nature Structural & Molecular Biology (2020)
-
TRF1 participates in chromosome end protection by averting TRF2-dependent telomeric R loops
Nature Structural & Molecular Biology (2018)