Abstract
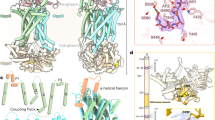
The outer membrane protein OprP mediates the transport of essential phosphate anions into the pathogenic bacterium Pseudomonas aeruginosa. Here we report the crystallographic structure of trimeric OprP at 1.9-Å resolution, revealing an unprecedented 9-residue arginine 'ladder' that spans from the extracellular surface down through a constriction zone where phosphate is coordinated. Lysine residues coat the inner periplasmic surface, creating an 'electropositive sink' that pulls the phosphates through the eyelet and into the cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
National Nosocomial Infections Surveillance System. Am. J. Infect. Control 32, 470–485 (2004).
Hancock, R.E. & Brinkman, F.S. Annu. Rev. Microbiol. 56, 17–38 (2002).
Wanner, B.L. J. C. Biochem. 51, 47–54 (1993).
Siehnel, R.J., Worobec, E.A. & Hancock, R.E. Mol. Microbiol. 2, 347–352 (1988).
Sukhan, A. & Hancock, R.E. J. Biol. Chem. 271, 21239–21242 (1996).
Benz, R., Egli, C. & Hancock, R.E. Biochim. Biophys. Acta 1149, 224–230 (1993).
Sukhan, A. & Hancock, R.E. J. Bacteriol. 177, 4914–4920 (1995).
Cowan, S.W. et al. Nature 358, 727–733 (1992).
Schulz, G.E. Biochim. Biophys. Acta 1565, 308–317 (2002).
Schirmer, T., Keller, T.A., Wang, Y.F. & Rosenbusch, J.P. Science 267, 512–514 (1995).
Zachariae, U., Koumanov, A., Engelhardt, H. & Karshikoff, A. Protein Sci. 11, 1309–1319 (2002).
Benz, R. & Hancock, R.E. J. Gen. Physiol. 89, 275–295 (1987).
Doyle, D.A. Eur. Biophys. J. 33, 175–179 (2004).
Hancock, R.E. & Benz, R. Biochim. Biophys. Acta 860, 699–707 (1986).
Acknowledgements
We thank C. Egli and N. Martin for valuable discussion and the staff at the Advanced Light Source beamline 8.2.2 for data collection time and assistance. T.F.M. is supported by a Michael Smith Foundation for Health Research (MSFHR) postdoctoral fellowship. N.C.J.S. thanks the Howard Hughes International Scholar program and the Canadian Institutes of Health Research (CIHR) for funding and the Canadian Foundation for Innovation and the MSFHR for infrastructure support. R.E.W.H. thanks the CIHR for funding, the Canadian Cystic Fibrosis Foundation and the US Cystic Fibrosis Foundation. N.C.J.S. is a MSFHR Senior Scholar, CIHR Investigator and Howard Hughes Medical Institute International Scholar. R.E.W.H. is a CIHR Investigator.
Author information
Authors and Affiliations
Contributions
M.B. purified and crystallized proteins; T.F.M. performed crystal optimization and X-ray structure determination; T.F.M., R.E.W.H. and N.C.J.S. were involved in the structural analysis and discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Secondary structural elements of OprP. (PDF 222 kb)
Supplementary Table 1
Data collection, phasing and refinement statistics. (PDF 79 kb)
Rights and permissions
About this article
Cite this article
Moraes, T., Bains, M., Hancock, R. et al. An arginine ladder in OprP mediates phosphate-specific transfer across the outer membrane. Nat Struct Mol Biol 14, 85–87 (2007). https://doi.org/10.1038/nsmb1189
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1189
This article is cited by
-
Structural basis for chitin acquisition by marine Vibrio species
Nature Communications (2018)
-
Probing the binding affinities of imipenem and ertapenem for outer membrane carboxylate channel D1 (OccD1) from P. aeruginosa: simulation studies
Journal of Molecular Modeling (2017)
-
A transport equation for confined structures applied to the OprP, Gramicidin A, and KcsA channels
Journal of Computational Electronics (2015)
-
Nanotunnel in bakteriellen Membranen
BIOspektrum (2014)
-
Structural insight into OprD substrate specificity
Nature Structural & Molecular Biology (2007)