Abstract
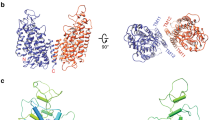
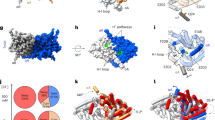
The ubiquitous CBS domains, which are found as part of cytoplasmic domains in the ClC family of chloride channels and transporters, have previously been identified as building blocks for regulatory nucleotide-binding sites. Here we report the structures of the cytoplasmic domain of the human transporter ClC-5 in complex with ATP and ADP. The nucleotides bind to a specific site in the protein. As determined by equilibrium dialysis, the affinities for ATP, ADP and AMP are in the high micromolar range. Point mutations that interfere with nucleotide binding change the transport behavior of a ClC-5 mutant expressed in Xenopus laevis oocytes. Our results establish the structural and energetic basis for the interaction of ClC-5 with nucleotides and provide a framework for future investigations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
19 January 2007
In the version of this article initially published, Figure 4 contained an error. The legend labels placed within Figure 4d were exchanged. The error has been corrected in the HTML and PDF versions of this article.
References
Jentsch, T.J., Neagoe, I. & Scheel, O. CLC chloride channels and transporters. Curr. Opin. Neurobiol. 15, 319–325 (2005).
Dutzler, R. The ClC family of chloride channels and transporters. Curr. Opin. Struct. Biol. 16, 439–446 (2006).
Dutzler, R., Campbell, E.B., Cadene, M., Chait, B.T. & MacKinnon, R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287–294 (2002).
Dutzler, R., Campbell, E.B. & MacKinnon, R. Gating the selectivity filter in ClC chloride channels. Science 300, 108–112 (2003).
Ignoul, S. & Eggermont, J. CBS domains: structure, function, and pathology in human proteins. Am. J. Physiol. Cell Physiol. 289, C1369–C1378 (2005).
Scott, J.W. et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 113, 274–284 (2004).
Vanoye, C.G. & George, A.L., Jr . Functional characterization of recombinant human ClC-4 chloride channels in cultured mammalian cells. J. Physiol. (Lond.) 539, 373–383 (2002).
Bennetts, B. et al. Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J. Biol. Chem. 280, 32452–32458 (2005).
Niemeyer, M.I. et al. Functional evaluation of human ClC-2 chloride channel mutations associated with idiopathic generalized epilepsies. Physiol. Genomics 19, 74–83 (2004).
Wellhauser, L. et al. Nucleotides bind to the C-terminus of ClC-5. Biochem. J. 398, 289–294 (2006).
Meyer, S. & Dutzler, R. Crystal structure of the cytoplasmic domain of the chloride channel ClC-0. Structure 14, 299–307 (2006).
Steinmeyer, K., Schwappach, B., Bens, M., Vandewalle, A. & Jentsch, T.J. Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J. Biol. Chem. 270, 31172–31177 (1995).
Schwake, M., Friedrich, T. & Jentsch, T.J. An internalization signal in ClC-5, an endosomal Cl-channel mutated in dent's disease. J. Biol. Chem. 276, 12049–12054 (2001).
Friedrich, T., Breiderhoff, T. & Jentsch, T.J. Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J. Biol. Chem. 274, 896–902 (1999).
Accardi, A. & Miller, C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature 427, 803–807 (2004).
Picollo, A. & Pusch, M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436, 420–423 (2005).
Scheel, O., Zdebik, A.A., Lourdel, S. & Jentsch, T.J. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436, 424–427 (2005).
Hardie, D.G. & Hawley, S.A. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 23, 1112–1119 (2001).
Adams, J. et al. Intrasteric control of AMPK via the gamma1 subunit AMP allosteric regulatory site. Protein Sci. 13, 155–165 (2004).
Walker, J.E., Saraste, M., Runswick, M.J. & Gay, N.J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1, 945–951 (1982).
Jiang, Y. et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522 (2002).
Miller, C. Open-state substructure of single chloride channels from Torpedo electroplax. Phil. Trans. R. Soc. Lond. B 299, 401–411 (1982).
Fong, P., Rehfeldt, A. & Jentsch, T.J. Determinants of slow gating in ClC-0, the voltage-gated chloride channel of Torpedo marmorata. Am. J. Physiol. 274, C966–C973 (1998).
Bykova, E.A., Zhang, X.D., Chen, T.Y. & Zheng, J. Large movement in the C terminus of CLC-0 chloride channel during slow gating. Nat. Struct. Mol. Biol. 13, 1115–1119 (2006).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 267, 307–326 (1997).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for X-ray crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Pape, T. & Schneider, T.R. HKL2MAP: a graphical user interface for phasing with SHELX programs. J. Appl. Cryst. 37, 843–844 (2004).
Schneider, T.R. & Sheldrick, G.M. Substructure solution with SHELXD. Acta Crystallogr. D Biol. Crystallogr. 58, 1772–1779 (2002).
Sheldrick, G.M. Macromolecular phasing with SHELXE. Z. Kristallographie 217, 644–650 (2002).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Brunger, A.T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Painter, J. & Merritt, E.A. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr. D Biol. Crystallogr. 62, 439–450 (2006).
Lorenz, C., Pusch, M. & Jentsch, T.J. Heteromultimeric CLC chloride channels with novel properties. Proc. Natl. Acad. Sci. USA 93, 13362–13366 (1996).
Acknowledgements
X-ray data were collected (MDC Berlin) at the Swiss Light Source of the Paul Scherrer Institute. We thank T. Jentsch (MDC Berlin) for providing the ClC-5 clone, S. Chesnov and P. Hunziker for help with mass spectrometry, B. Blattmann for help with crystal screening, P. Lindner for advice on equilibrium binding assays, E. Hänsenberger for preparation of the Xenopus oocytes, the staff of the X06SA beamline for their support during data collection and R. MacKinnon for comments on the manuscript. This work was supported by a grant from the Swiss National Science Foundation and the National Center of Competence in Research in Structural Biology program. S.M. is affiliated with the Molecular Life Sciences PhD Program of the University/ETH Zürich.
Author information
Authors and Affiliations
Contributions
S.M. carried out all experiments, S.S. assisted in protein preparation and binding assays, I.C.F. contributed to electrophysiology experiments and R.D. conceived and planned the experiments and interpreted the data. S.M. and R.D. jointly wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Sedimentation velocity data for the ClC-5 cytoplasmic domain. (PDF 336 kb)
Supplementary Fig. 2
Stereo view of experimental density of the nucleotide-binding region in the ClC-5 domain–ATP complex. (PDF 334 kb)
Supplementary Fig. 3
Competition of bound radiolabeled ATP. (PDF 11 kb)
Supplementary Fig. 4
Two-electrode voltage-clamp recordings of human ClC-5 in Xenopus oocytes. (PDF 179 kb)
Supplementary Fig. 5
Two-electrode voltage-clamp recordings of human ClC-5 mutants in Xenopus oocytes. (PDF 175 kb)
Supplementary Fig. 6
Two-electrode voltage-clamp recordings of human ClC-5 double mutants in Xenopus oocytes. (PDF 176 kb)
Rights and permissions
About this article
Cite this article
Meyer, S., Savaresi, S., Forster, I. et al. Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5. Nat Struct Mol Biol 14, 60–67 (2007). https://doi.org/10.1038/nsmb1188
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1188
This article is cited by
-
ATP-dependent modulation of MgtE in Mg2+ homeostasis
Nature Communications (2017)
-
Crystal structure of a hypothetical protein, TTHA0829 from Thermus thermophilus HB8, composed of cystathionine-β-synthase (CBS) and aspartate-kinase chorismate-mutase tyrA (ACT) domains
Extremophiles (2016)
-
Expression patterns of As-ClC gene of Artemia sinica in early development and under salinity stress
Molecular Biology Reports (2013)
-
Transgenic overexpression of UIP1, an interactor of the 3′ untranslated region of the Rubisco small subunit mRNA, increases rice tolerance to drought
Plant Biotechnology Reports (2013)
-
Regulation of ClC-2 gating by intracellular ATP
Pflügers Archiv - European Journal of Physiology (2013)