Abstract
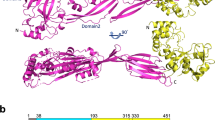
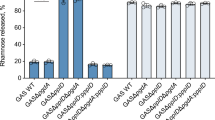
The ability of pathogenic bacteria to recognize host glycans is often essential to their virulence. Here we report structure-function studies of previously uncharacterized glycogen-binding modules in the surface-anchored pullulanases from Streptococcus pneumoniae (SpuA) and Streptococcus pyogenes (PulA). Multivalent binding to glycogen leads to a strong interaction with alveolar type II cells in mouse lung tissue. X-ray crystal structures of the binding modules reveal a novel fusion of tandem modules into single, bivalent functional domains. In addition to indicating a structural basis for multivalent attachment, the structure of the SpuA modules in complex with carbohydrate provides insight into the molecular basis for glycogen specificity. This report provides the first evidence that intracellular lung glycogen may be a novel target of pathogenic streptococci and thus provides a rationale for the identification of the streptococcal α-glucan–metabolizing machinery as virulence factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Manco, S. et al. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect. Immun. 74, 4014–4020 (2006).
Soong, G. et al. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J. Clin. Invest. 116, 2297–2305 (2006).
Hava, D.L. & Camilli, A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45, 1389–1406 (2002).
Boraston, A.B., Bolam, D.N., Gilbert, H.J. & Davies, G.J. Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem. J. 382, 769–781 (2004).
Lammerts van Bueren, A., Finn, R., Ausio, J. & Boraston, A.B. Alpha-glucan recognition by a new family of carbohydrate-binding modules found primarily in bacterial pathogens. Biochemistry 43, 15633–15642 (2004).
Tomasz, A. New faces of an old pathogen: emergence and spread of multidrug-resistant Streptococcus pneumoniae. Am. J. Med. 107, 55S–62S (1999).
Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13, 470–511 (2000).
Cengiz, A.B. et al. Fatal necrotizing pneumonia caused by group A streptococcus. J. Paediatr. Child Health 40, 69–71 (2004).
Morozumi, M. et al. Simultaneous detection of pathogens in clinical samples from patients with community-acquired pneumonia by real-time PCR with pathogen-specific molecular beacon probes. J. Clin. Microbiol. 44, 1440–1446 (2006).
Shelburne, S.A., III et al. Maltodextrin utilization plays a key role in the ability of group A Streptococcus to colonize the oropharynx. Infect. Immun. 74, 4605–4614 (2006).
Bongaerts, R.J., Heinz, H.P., Hadding, U. & Zysk, G. Antigenicity, expression, and molecular characterization of surface-located pullulanase of Streptococcus pneumoniae. Infect. Immun. 68, 7141–7143 (2000).
Hytonen, J., Haataja, S. & Finne, J. Streptococcus pyogenes glycoprotein-binding strepadhesin activity is mediated by a surface-associated carbohydrate-degrading enzyme, pullulanase. Infect. Immun. 71, 784–793 (2003).
Hytonen, J., Haataja, S. & Finne, J. Use of flow cytometry for the adhesion analysis of Streptococcus pyogenes mutant strains to epithelial cells: investigation of the possible role of surface pullulanase and cysteine protease, and the transcriptional regulator Rgg. BMC Microbiol. 6, 18 (2006).
Kriegshauser, G. & Liebl, W. Pullulanase from the hyperthermophilic bacterium Thermotoga maritima: purification by beta-cyclodextrin affinity chromatography. J. Chromatogr. B Biomed. Sci. Appl. 737, 245–251 (2000).
Mikami, B. et al. Crystal structure of pullulanase: evidence for parallel binding of oligosaccharides in the active site. J. Mol. Biol. 359, 690–707 (2006).
Sorimachi, K., Le Gal-Coeffet, M.F., Williamson, G., Archer, D.B. & Williamson, M.P. Solution structure of the granular starch binding domain of Aspergillus niger glucoamylase bound to beta-cyclodextrin. Structure 5, 647–661 (1997).
Boraston, A.B. et al. A structural and functional analysis of alpha-glucan recognition by family 25 and 26 carbohydrate-binding modules reveals a conserved mode of starch recognition. J. Biol. Chem. 281, 587–598 (2006).
Boraston, A.B., Kwan, E., Chiu, P., Warren, R.A. & Kilburn, D.G. Recognition and hydrolysis of noncrystalline cellulose. J. Biol. Chem. 278, 6120–6127 (2003).
Lammerts van Bueren, A. & Boraston, A.B. The structural basis of alpha-glucan recognition by a family 41 carbohydrate-binding module from Thermotoga maritima. J. Mol. Biol. published online 11 October 2006 (doi:10.1016/j.jmb.2006.10.018).
Sorimachi, K. et al. Solution structure of the granular starch binding domain of glucoamylase from Aspergillus niger by nuclear magnetic resonance spectroscopy. J. Mol. Biol. 259, 970–987 (1996).
Sakon, J., Irwin, D., Wilson, D.B. & Karplus, P.A. Structure and mechanism of endo/exocellulase E4 from Thermomonospora fusca. Nat. Struct. Biol. 4, 810–818 (1997).
Weaver, T.E. & Conkright, J.J. Function of surfactant proteins B and C. Annu. Rev. Physiol. 63, 555–578 (2001).
Glasser, S.W. et al. Pneumonitis and emphysema in sp-C gene targeted mice. J. Biol. Chem. 278, 14291–14298 (2003).
Cundell, D.R. & Tuomanen, E.I. Receptor specificity of adherence of Streptococcus pneumoniae to human type-II pneumocytes and vascular endothelial cells in vitro. Microb. Pathog. 17, 361–374 (1994).
Kadioglu, A. et al. Use of green fluorescent protein in visualisation of pneumococcal invasion of broncho-epithelial cells in vivo. FEMS Microbiol. Lett. 194, 105–110 (2001).
Ridsdale, R. & Post, M. Surfactant lipid synthesis and lamellar body formation in glycogen-laden type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L743–L751 (2004).
Rannels, S.R., Rannels, S.L., Sneyd, J.G. & Loten, E.G. Fetal lung development in rats with a glycogen storage disorder. Am. J. Physiol. 260, L419–L427 (1991).
Rooney, S.A. Regulation of surfactant secretion. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129, 233–243 (2001).
Jounblat, R. et al. Binding and agglutination of Streptococcus pneumoniae by human surfactant protein D (SP-D) vary between strains, but SP-D fails to enhance killing by neutrophils. Infect. Immun. 72, 709–716 (2004).
Barik, S. Site-directed mutagenesis in vitro by megaprimer PCR. Methods Mol. Biol. 57, 203–215 (1996).
Boraston, A.B. et al. Binding specificity and thermodynamics of a family 9 carbohydrate-binding module from Thermotoga maritima xylanase 10A. Biochemistry 40, 6240–6247 (2001).
Ficko-Blean, E. & Boraston, A.B. Cloning, recombinant production, crystallization and preliminary X-ray diffraction studies of a family 84 glycoside hydrolase from Clostridium perfringens. Acta Crystallograph. Sect. F Struct. Biol. Cryst. Commun. 61, 834–836 (2005).
Mach, H., Middaugh, C.R. & Lewis, R.V. Statistical determination of the average values of the extinction coefficients of tryptophan and tyrosine in native proteins. Anal. Biochem. 200, 74–80 (1992).
Tomme, P., Boraston, A., Kormos, J.M., Warren, R.A. & Kilburn, D.G. Affinity electrophoresis for the identification and characterization of soluble sugar binding by carbohydrate-binding modules. Enzyme Microb. Technol. 27, 453–458 (2000).
Sigurskjold, B.W., Altman, E. & Bundle, D.R. Sensitive titration microcalorimetric study of the binding of Salmonella O-antigenic oligosaccharides by a monoclonal antibody. Eur. J. Biochem. 197, 239–246 (1991).
Pflugrath, J.W. The finer things in X-ray diffraction data collection. Acta Crystallogr. D Biol. Crystallogr. 55, 1718–1725 (1999).
Schneider, T.R. & Sheldrick, G.M. Substructure solution with SHELXD. Acta Crystallogr. D Biol. Crystallogr. 58, 1772–1779 (2002).
Evans, G. & Bricogne, G. Triiodide derivatization and combinatorial counter-ion replacement: two methods for enhancing phasing signal using laboratory Cu Kalpha X-ray equipment. Acta Crystallogr. D Biol. Crystallogr. 58, 976–991 (2002).
Cowtan, K.D. & Zhang, K.Y. Density modification for macromolecular phase improvement. Prog. Biophys. Mol. Biol. 72, 245–270 (1999).
Perrakis, A., Morris, R. & Lamzin, V.S. Automated protein model building combined with iterative structure refinement. Nat. Struct. Biol. 6, 458–463 (1999).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Vagin, A. & Teplyakov, A. An approach to multi-copy search in molecular replacement. Acta Crystallogr. D Biol. Crystallogr. 56, 1622–1624 (2000).
Brunger, A.T. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355, 472–475 (1992).
Read, R.J. Improved Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A 42, 140–149 (1986).
Acknowledgements
We thank R. Chow for providing access to and assistance with the confocal imaging microscope. This work was supported by grants from the Canadian Institutes of Health Research and Natural Sciences and Engineering Council of Canada (A.B.B. and R.D.B.). A.L.v.B. is supported by doctoral fellowships from the Michael Smith Foundation for Health Research and the Natural Sciences and Engineering Research Council of Canada. A.B.B. is a Canada Research Chair in Molecular Interactions.
Author information
Authors and Affiliations
Contributions
A.L.v.B., cloning, protein production and purification, binding studies, crystallization, structure solution and analysis and figure and manuscript preparation; M.H., cloning and protein production and purification; D.W., lung section preparation and probing; R.D.B., fluorescence and confocal microscopic imaging and figure preparation; A.B.B., principle investigator and figure and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
van Bueren, A., Higgins, M., Wang, D. et al. Identification and structural basis of binding to host lung glycogen by streptococcal virulence factors. Nat Struct Mol Biol 14, 76–84 (2007). https://doi.org/10.1038/nsmb1187
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1187
This article is cited by
-
The impact of N-terminal nonessential domains on the enzymological properties of the pullulanase from a marine Bacillus megaterium
Biotechnology Letters (2019)
-
Structure and function of α-glucan debranching enzymes
Cellular and Molecular Life Sciences (2016)
-
Identification of Potential Vaccine Candidates Against Streptococcus pneumoniae by Reverse Vaccinology Approach
Applied Biochemistry and Biotechnology (2014)
-
Prise en charge des pneumonies graves à pneumocoque — Pneumonies communautaires aiguës sévères à Streptococcus pneumoniae (PAC Sp): rôle de l’hôte et des facteurs de virulence bactérienne
Réanimation (2011)
-
New adhesin functions of surface-exposed pneumococcal proteins
BMC Microbiology (2010)