Abstract
The small-subunit processome represents the earliest stable precursor of the eukaryotic small ribosomal subunit. Here we present the cryo-EM structure of the Saccharomyces cerevisiae small-subunit processome at an overall resolution of 3.8 Å, which provides an essentially complete near-atomic model of this assembly. In this nucleolar superstructure, 51 ribosome-assembly factors and two RNAs encapsulate the 18S rRNA precursor and 15 ribosomal proteins in a state that precedes pre-rRNA cleavage at site A1. Extended flexible proteins are employed to connect distant sites in this particle. Molecular mimicry and steric hindrance, as well as protein- and RNA-mediated RNA remodeling, are used in a concerted fashion to prevent the premature formation of the central pseudoknot and its surrounding elements within the small ribosomal subunit.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Woolford, J.L. Jr. & Baserga, S.J. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 195, 643–681 (2013).
Mougey, E.B. et al. The terminal balls characteristic of eukaryotic rRNA transcription units in chromatin spreads are rRNA processing complexes. Genes Dev. 7, 1609–1619 (1993).
Osheim, Y.N. et al. Pre-18S ribosomal RNA is structurally compacted into the SSU processome prior to being cleaved from nascent transcripts in Saccharomyces cerevisiae. Mol. Cell 16, 943–954 (2004).
Dragon, F. et al. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417, 967–970 (2002).
Fatica, A., Tollervey, D. & Dlakić, M. PIN domain of Nob1p is required for D-site cleavage in 20S pre-rRNA. RNA 10, 1698–1701 (2004).
Pérez-Fernández, J., Román, A., De Las Rivas, J., Bustelo, X.R. & Dosil, M. The 90S preribosome is a multimodular structure that is assembled through a hierarchical mechanism. Mol. Cell. Biol. 27, 5414–5429 (2007).
Chaker-Margot, M., Hunziker, M., Barandun, J., Dill, B.D. & Klinge, S. Stage-specific assembly events of the 6-MDa small-subunit processome initiate eukaryotic ribosome biogenesis. Nat. Struct. Mol. Biol. 22, 920–923 (2015).
Zhang, L., Wu, C., Cai, G., Chen, S. & Ye, K. Stepwise and dynamic assembly of the earliest precursors of small ribosomal subunits in yeast. Genes Dev. 30, 718–732 (2016).
Hunziker, M. et al. UtpA and UtpB chaperone nascent pre-ribosomal RNA and U3 snoRNA to initiate eukaryotic ribosome assembly. Nat. Commun. 7, 12090 (2016).
Marmier-Gourrier, N., Cléry, A., Schlotter, F., Senty-Ségault, V. & Branlant, C. A second base pair interaction between U3 small nucleolar RNA and the 5′-ETS region is required for early cleavage of the yeast pre-ribosomal RNA. Nucleic Acids Res. 39, 9731–9745 (2011).
Dutca, L.M., Gallagher, J.E.G. & Baserga, S.J. The initial U3 snoRNA:pre-rRNA base pairing interaction required for pre-18S rRNA folding revealed by in vivo chemical probing. Nucleic Acids Res. 39, 5164–5180 (2011).
Kornprobst, M. et al. Architecture of the 90S pre-ribosome: a structural view on the birth of the eukaryotic ribosome. Cell 166, 380–393 (2016).
Chaker-Margot, M., Barandun, J., Hunziker, M. & Klinge, S. Architecture of the yeast small subunit processome. Science 355, eaal1880 (2017).
Sun, Q. et al. Molecular architecture of the 90S small subunit pre-ribosome. eLife 6, e22086 (2017).
Talkish, J. et al. Disruption of ribosome assembly in yeast blocks cotranscriptional pre-rRNA processing and affects the global hierarchy of ribosome biogenesis. RNA 22, 852–866 (2016).
Kos-Braun, I.C., Jung, I. & Koš, M. Tor1 and CK2 kinases control a switch between alternative ribosome biogenesis pathways in a growth-dependent manner. PLoS Biol. 15, e2000245 (2017).
Makarova, K.S., Wolf, Y.I., Mekhedov, S.L., Mirkin, B.G. & Koonin, E.V. Ancestral paralogs and pseudoparalogs and their role in the emergence of the eukaryotic cell. Nucleic Acids Res. 33, 4626–4638 (2005).
Khoshnevis, S. et al. The DEAD-box protein Rok1 orchestrates 40S and 60S ribosome assembly by promoting the release of Rrp5 from pre-40S ribosomes to allow for 60S maturation. PLoS Biol. 14, e1002480 (2016).
Torchet, C. & Jacq, C. Hermann-Le Denmat, S. Two mutant forms of the S1/TPR-containing protein Rrp5p affect the 18S rRNA synthesis in Saccharomyces cerevisiae. RNA 4, 1636–1652 (1998).
Eppens, N.A., Rensen, S., Granneman, S., Raué, H.A. & Venema, J. The roles of Rrp5p in the synthesis of yeast 18S and 5.8S rRNA can be functionally and physically separated. RNA 5, 779–793 (1999).
Lin, J., Lu, J., Feng, Y., Sun, M. & Ye, K. An RNA-binding complex involved in ribosome biogenesis contains a protein with homology to tRNA CCA-adding enzyme. PLoS Biol. 11, e1001669 (2013).
Zheng, S., Lan, P., Liu, X. & Ye, K. Interaction between ribosome assembly factors Krr1 and Faf1 is essential for formation of small ribosomal subunit in yeast. J. Biol. Chem. 289, 22692–22703 (2014).
Zhang, L., Lin, J. & Ye, K. Structural and functional analysis of the U3 snoRNA binding protein Rrp9. RNA 19, 701–711 (2013).
Kühn, H. et al. The Noc-domain containing C-terminus of Noc4p mediates both formation of the Noc4p-Nop14p submodule and its incorporation into the SSU processome. PLoS One 4, e8370 (2009).
Lee, S.J. & Baserga, S.J. Imp3p and Imp4p, two specific components of the U3 small nucleolar ribonucleoprotein that are essential for pre-18S rRNA processing. Mol. Cell. Biol. 19, 5441–5452 (1999).
Zheng, S. & Ye, K. Purification, crystallization and preliminary X-ray diffraction analysis of Imp3 in complex with an Mpp10 peptide involved in yeast ribosome biogenesis. Acta Crystallogr. F Struct. Biol. Commun. 70, 918–921 (2014).
Thoms, M. et al. The exosome is recruited to RNA substrates through specific adaptor proteins. Cell 162, 1029–1038 (2015).
Zhu, J., Liu, X., Anjos, M., Correll, C.C. & Johnson, A.W. Utp14 recruits and activates the RNA helicase Dhr1 to undock U3 snoRNA from the pre-ribosome. Mol. Cell Biol. 36, 965–978 (2016).
Sardana, R. et al. The DEAH-box helicase Dhr1 dissociates U3 from the pre-rRNA to promote formation of the central pseudoknot. PLoS Biol. 13, e1002083 (2015).
Gelperin, D., Horton, L., Beckman, J., Hensold, J. & Lemmon, S.K. Bms1p, a novel GTP-binding protein, and the related Tsr1p are required for distinct steps of 40S ribosome biogenesis in yeast. RNA 7, 1268–1283 (2001).
Delprato, A. et al. Crucial role of the Rcl1p-Bms1p interaction for yeast pre-ribosomal RNA processing. Nucleic Acids Res. 42, 10161–10172 (2014).
Mitchell, P. Rrp47 and the function of the Sas10/C1D domain. Biochem. Soc. Trans. 38, 1088–1092 (2010).
Ben-Shem, A. et al. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 334, 1524–1529 (2011).
Sondalle, S.B. & Baserga, S.J. Human diseases of the SSU processome. Biochim. Biophys. Acta 1842, 758–764 (2014).
Robson, A., Owens, N.D.L., Baserga, S.J., Khokha, M.K. & Griffin, J.N. Expression of ribosomopathy genes during Xenopus tropicalis embryogenesis. BMC Dev. Biol. 16, 38 (2016).
Paolini, N.A. et al. A ribosomopathy reveals decoding defective ribosomes driving human dysmorphism. Am. J. Hum. Genet. 100, 506–522 (2017).
Marneros, A.G. BMS1 is mutated in aplasia cutis congenita. PLoS Genet. 9, e1003573 (2013).
Lebaron, S. et al. Proofreading of pre-40S ribosome maturation by a translation initiation factor and 60S subunits. Nat. Struct. Mol. Biol. 19, 744–753 (2012).
Mastronarde, D.N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S.Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Kimanius, D., Forsberg, B.O., Scheres, S.H. & Lindahl, E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. eLife 5, e.18722 (2016).
Kucukelbir, A., Sigworth, F.J. & Tagare, H.D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Kelley, L.A., Mezulis, S., Yates, C.M., Wass, M.N. & Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E.F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Shi, Y. et al. Structural characterization by cross-linking reveals the detailed architecture of a coatomer-related heptameric module from the nuclear pore complex. Mol. Cell. Proteomics 13, 2927–2943 (2014).
Shi, Y. et al. A strategy for dissecting the architectures of native macromolecular assemblies. Nat. Methods 12, 1135–1138 (2015).
Yang, B. et al. Identification of cross-linked peptides from complex samples. Nat. Methods 9, 904–906 (2012).
Combe, C.W., Fischer, L. & Rappsilber, J. xiNET: cross-link network maps with residue resolution. Mol. Cell. Proteomics 14, 1137–1147 (2015).
Acknowledgements
We thank M. Ebrahim and J. Sotiris for outstanding support with data collection at the Evelyn Gruss Lipper Cryo-EM resource center at The Rockefeller University. We further thank T. Walz, G. Alushin and Y. Shi for helpful discussions. J.B. is supported by an EMBO long-term fellowship (ALTF 51-2014) and a Swiss National Science Foundation fellowship (155515), M.C.-M. is supported by a postgraduate scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC) and S.K. is supported by the Robertson Foundation, the Alfred P. Sloan Foundation, the Irma T. Hirschl Trust, the Alexandrine and Alexander L. Sinsheimer Fund, the Human Frontier Science Program Career Development Award and the NIH New Innovator Award (1DP2GM123459). B.T.C. is supported by National Institute of Health grant nos. P41GM103314 and P41GM109824.
Author information
Authors and Affiliations
Contributions
S.K. established purification conditions, and M.C.-M. and J.B. acquired cryo-EM data. K.R.M. performed MS experiments and analyzed the resulting data with B.T.C. J.B., M.C.-M., M.H. and S.K. determined the cryo-EM structure of the yeast SSU processome, built the atomic model, interpreted the results and wrote the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
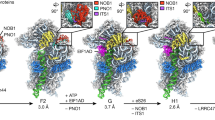
Supplementary Figure 1 Alternative processing pathways of the small eukaryotic ribosomal subunit.
Schematic representation of the 35S pre-rRNA with external transcribed spacer regions (5’ ETS, 3’ ETS), internal transcribed spacer regions (ITS1, ITS2) and ribosomal RNAs (18S, 5.8S, 25S). The positions of RNA cleavage sites are indicated above. Small subunit processing intermediates resulting from A2 cleavage (in rich medium) and A3 cleavage (under starvation conditions) are shown. The processing state of the SSU processome described in this manuscript is highlighted in a pink box. Key cleavage events are highlighted in red with the particle (SSU processome), protein complex (RNase MRP) or individual protein (Nob1) responsible for cleavage indicated in parentheses.
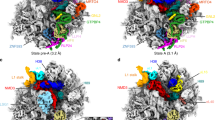
Supplementary Figure 2 Cryo-EM data processing strategy.
10,029 micrographs were collected in four independent sessions (Datasets 1-4) and aligned using MotionCor2 (ref. 40) with dose weighting. Manual inspection and elimination of low quality micrographs reduced this number to 8,406 used for particle picking in RELION-2.0 (ref. 42) (Autopicking and extensive manual cleanup). 3D classification with five classes yielded 2 good classes containing 284,213 particles. Overall 3D refinement resulted in a reconstruction at a resolution of 3.8 Å. Focused refinement was performed for the core (3.6 Å) and the 3’ domain (4.1 Å). Focused and iterative 3D classification using a head mask (pink dashed line) or a central domain mask improved maps for these regions.
Supplementary Figure 3 Overall and local resolution estimation of all obtained cryo-EM maps.
Overall and local resolution estimation of (a) the overall map at 3.8 Å (overall map 1), (b) the core focused map at 3.6 Å (core map), (c) the overall map with focus on the head region at 4.1 Å (overall map 2), (d) 3’ domain and UtpA focused map at 4.1 Å (3’ domain map) and (e) the central domain focused map at 7.2 Å (central domain map). a-e The left panel shows Fourier Shell Correlation (FSC) curves for the unmasked (dashed black line), phase-randomized (solid grey line), the masked (dashed grey line) and the corrected map (solid black line). FSC curves calculated between the final model and map (light-blue), model2 and half map 1 (FSC test; red) or half map 2 (FSC work; yellow dashed line) are shown. An FSC value of 0.143 is indicated by a thin black line. Three views related by a 120-degree rotation of the obtained cryo-EM map are shown colored according to local resolution. The fourth density panel shows a slab view visualizing the resolution in the center. Local resolution was calculated using Resmap43.
Supplementary Figure 4 Representative electron density of selected protein and RNA elements.
(a) Cryo-EM density and corresponding models of selected protein segments are shown. Labels below the density indicate the subunit and the corresponding residue-range visualized. While most elements show representative density from the core region of the SSU processome, Utp6 is located in the periphery and Utp15 is located towards the bottom of the particle. (b-d) Models of (b) the 5’ ETS helices IV and VI, and the A1 cleavage site, (c) the 18S rRNA 5’ and 3’ domains, and helix 44, (d) the U3 snoRNA box A, 5’ and 3’ hinges and Box C’/D are depicted within their respective cryo-EM densities.
Supplementary Figure 5 The helical repeat proteins Utp20 and Rrp5 chaperone rRNA in the 5’- and central domain.
(a and b) Two views of a composite cryo-EM density map consisting of the 6 Å low-pass filtered overall map 2 and the 7.2 Å central domain map. The density is colored as in Figure 1 but with the pre-18S RNA colored in pale-green. Helices (h8, h10, h24, h44) and expansion segments (ES3A, ES3B) of the 18S rRNA are labeled next to the corresponding density. In (b) the density for the tetratricopeptide repeat (TPR) of Rrp5 is shown transparent with the docked crystal structure (PDB 5C9S). (c) Cryo-EM density from the central domain map with molecular fit of the TPR repeat crystal structure of Rrp5 (PDB 5C9S), shown as cartoon. The concave interface serves as binding platform for 18S rRNA helix 24 (h24, in green).
Supplementary Figure 6 Overview and structural analysis of the DSS cross-linking mass spectrometry data.
(a) Two-dimensional visualization of all inter-protein DSS cross-links obtained for the SSU processome sample generated with xiNET52. Protein subunits are represented as spheres. The size of each sphere is proportional to the molecular weight of the corresponding protein. Subunits belonging to complexes or those forming a structural unit are highlighted with the same color. The thickness of the line connecting two subunits is proportional to the number of shared cross-links. All U-three-proteins (Utp) are labeled with their respective number. (b) Cross-links plotted onto the structure of the SSU processome shown as direct connection between the Cα of individual lysine residues. All Cα atoms found in the cross-linking analysis are shown as spheres and are colored according to Figure 1. In cases where two copies of a protein are present (Kre33, Emg1, and Nop1), the shorter cross-link is displayed. Conformational flexibility of the central domain and a reconstruction of this domain based on only a small subpopulation of the data (15%) may explain the high abundance of cross-links with longer distances in this region. These cross-links may result from other conformational states of the central domain. (c) Histogram of all Cα cross-link distances in Å. 87.2% of all cross-link distances are within 32 Å. (d) SDS-PAGE analysis of a purified SSU processome sample cross-linked with increasing concentrations of DSS. The gel region and DSS concentration used for mass spectrometry analysis experiments are highlighted in green.
Supplementary Figure 7 Structural comparison of L1-domain containing proteins.
(a) Structure of Utp30 (deep-teal) and Rrt14 (light-green) with pre-18S RNA (white) and 5’ ETS (yellow) in the SSU processome. (b) Structure of Cic1 (purple) and Nop15 (orange) with ITS2 (white) in the Nog2 particle (PDB 3JCT) (c) Structure of Thermus thermophilus L1 in complex with 23S rRNA (PDB 3U4M).
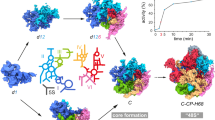
Supplementary Figure 8 Structural analysis of Bms1 and its interaction partners.
(a) Overview of Bms1 and its binding partners within the SSU processome. Proteins are colored as in Figure 1 with a transparent outline of the SSU processome in white. (b) Architecture of the Bms1-Rcl1 complex with Bms1 domains I-IV, the Kre33-binding domain (Kre33-BD) and the Rcl1-binding domain (Rcl1-BD) color-coded in shades of violet. The Bms1 C-terminal domain (CTD) is highlighted in light-brown. (c) Two views of the interactions of Bms1 with other SSU processome subunits. Only the most N-terminal domains of one Kre33 monomer (yellow) are shown.
Supplementary Figure 9 Secondary structure diagram of RNAs in the SSU processome.
Individual nucleotides of RNAs are indicated with their base pairing interactions. 5’ ETS (grey), 18S (black) and U3 snoRNA (light-blue) are shown. Regions of the 18S rRNA that have been remodeled in the SSU processome are highlighted in red.
Supplementary Figure 10 Structural context of proteins implicated in ribosomopathies.
(a-c) Three views of the SSU processome (grey), related by 120 degree rotations. Proteins associated with ribosomopathies are highlighted in colors as follows: Emg1 (yellow, orange), Utp4 (blue), rpS23 (pale-yellow), rpS14 (red), rpS7 (green), Bms1 (violet), and Utp14 (cyan). (d and e) Comparative views of rpS23 (pale-yellow) in the context of the SSU processome (d) and the mature small subunit (e). (d) View of rpS23 (pale-yellow) in the context of the SSU processome. Utp11 (blue), Bms1 (violet) and pre-18S rRNA (light-grey) are shown. A loop of rpS23 (residues 58-70) is shown in orange. Arginine 69 (R69) of rpS23 and its interaction partners are depicted as sticks, G568 of pre-18S rRNA is highlighted in green. (e) View of rpS23 in the context of the mature small ribosomal subunit (PDB 4V88). RpS23 (pale-yellow), rpS30 (blue) and 18S rRNA (light-grey) are shown. A loop of rpS23 (residues 58-70) is colored orange. Arginine 69 (R69) and Aspartate 116 (D116) are depicted as sticks and nucleotide G568 is highlighted in green. (f) Structural context of Bms1 arginine 833 (human R930) in the SSU processome. Bms1 (violet) and rpS23 (pale-yellow) are shown. Arginine 833 and its interacting residues are shown as sticks. (g) Superposition of rpS23 in the context of the mature small ribosomal subunit (pale-green) and the SSU processome (pale-yellow). Loops that adopt different conformations (loop1 and loop2) are highlighted in darker shades.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Supplementary Table 1 and Supplementary Notes 1–21. (PDF 28562 kb)
Supplementary Data Set 1
DSS-cross-links of the SSU processome. (XLSX 49 kb)
Supplementary Data Set 2
PyMOL session for the structural analysis of the SSU processome. (ZIP 11674 kb)
360° view of the cryo-EM reconstruction of the S. cerevisiae SSU processome.
360° rotation of a composite cryo-EM map consisting of the 3.6 Å core, the 4.1 Å head-focused, the 4.1 Å 3′ domain and the 7.2 Å central domain maps. Densities for SSU processome components are color-coded. The rotation is paused at the same views as shown in Fig. 1 (0°, 120° and 240°) and labels for individual subunits are displayed. Subunits of complexes are shown in shades of blue (UtpA), red (UtpB), purple (U3 snoRNP), brown (Nop14/Noc4) and light-pink (Bms1-Rcl1). Ribosomal proteins are depicted in shades of grey. RNAs are colored in yellow (5′ ETS), red (U3 snoRNA) and white (pre-18S rRNA). (MP4 30218 kb)
Rights and permissions
About this article
Cite this article
Barandun, J., Chaker-Margot, M., Hunziker, M. et al. The complete structure of the small-subunit processome. Nat Struct Mol Biol 24, 944–953 (2017). https://doi.org/10.1038/nsmb.3472
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3472
This article is cited by
-
Visualizing the nucleoplasmic maturation of human pre-60S ribosomal particles
Cell Research (2023)
-
Sequence-specific remodeling of a topologically complex RNP substrate by Spb4
Nature Structural & Molecular Biology (2022)
-
Artificial intelligence-assisted cryoEM structure of Bfr2-Lcp5 complex observed in the yeast small subunit processome
Communications Biology (2022)
-
Eukaryotic Box C/D methylation machinery has two non-symmetric protein assembly sites
Scientific Reports (2021)
-
The nucleolar DExD/H protein Hel66 is involved in ribosome biogenesis in Trypanosoma brucei
Scientific Reports (2021)